Abstract
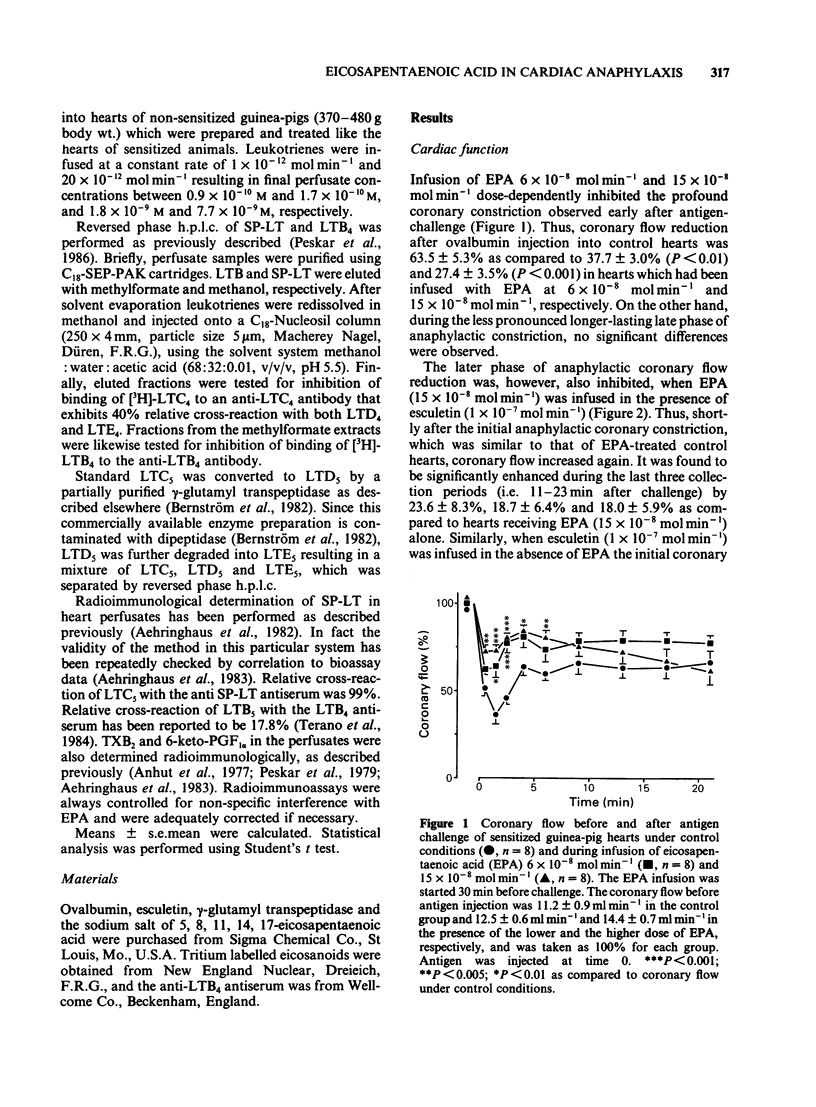
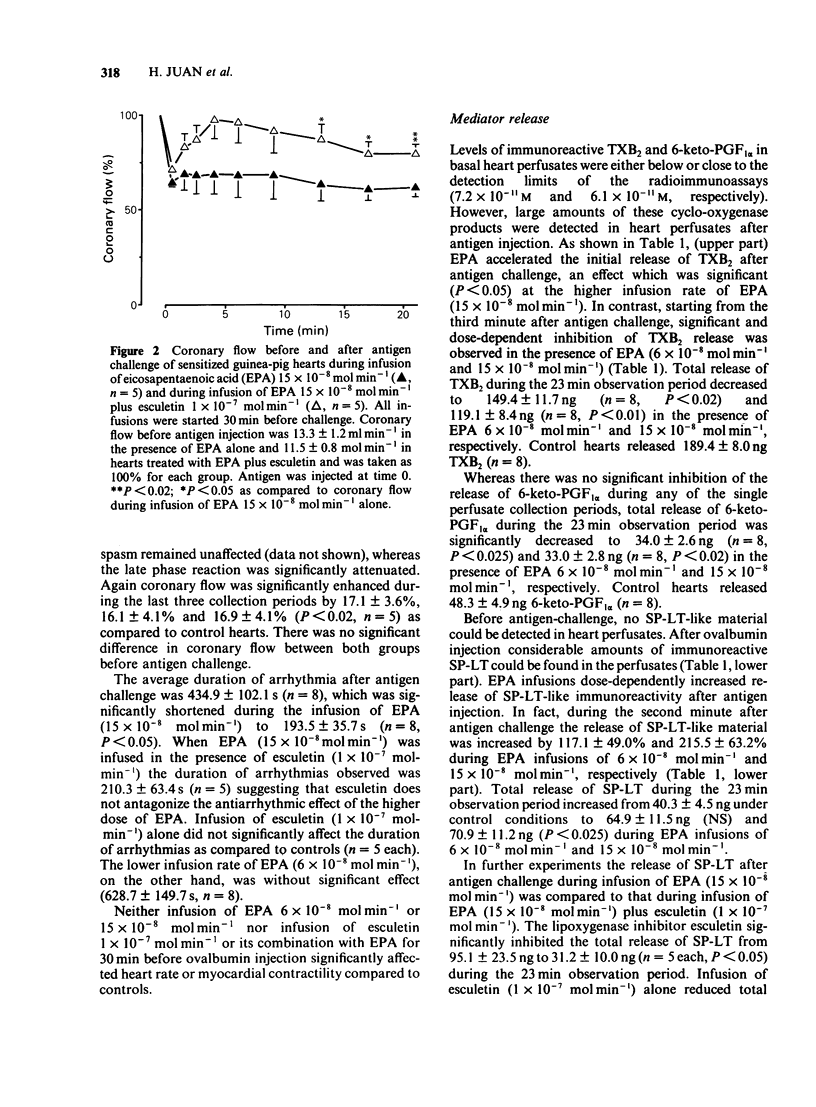
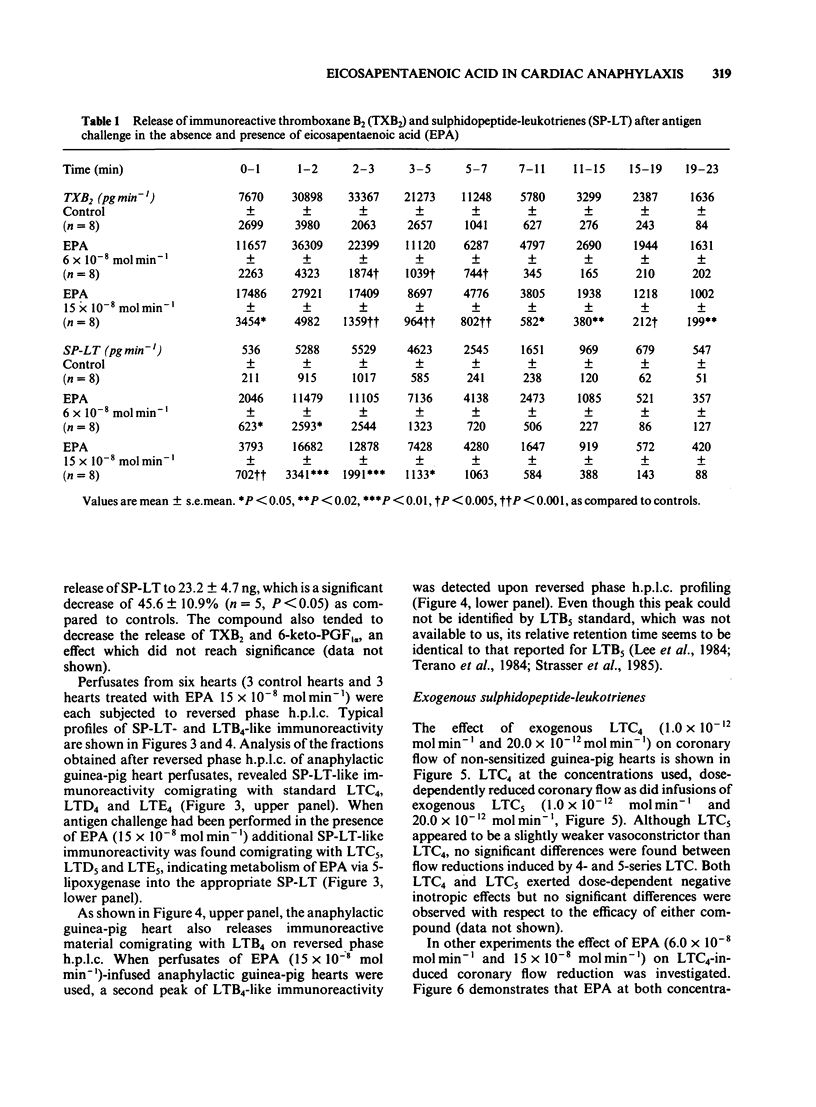
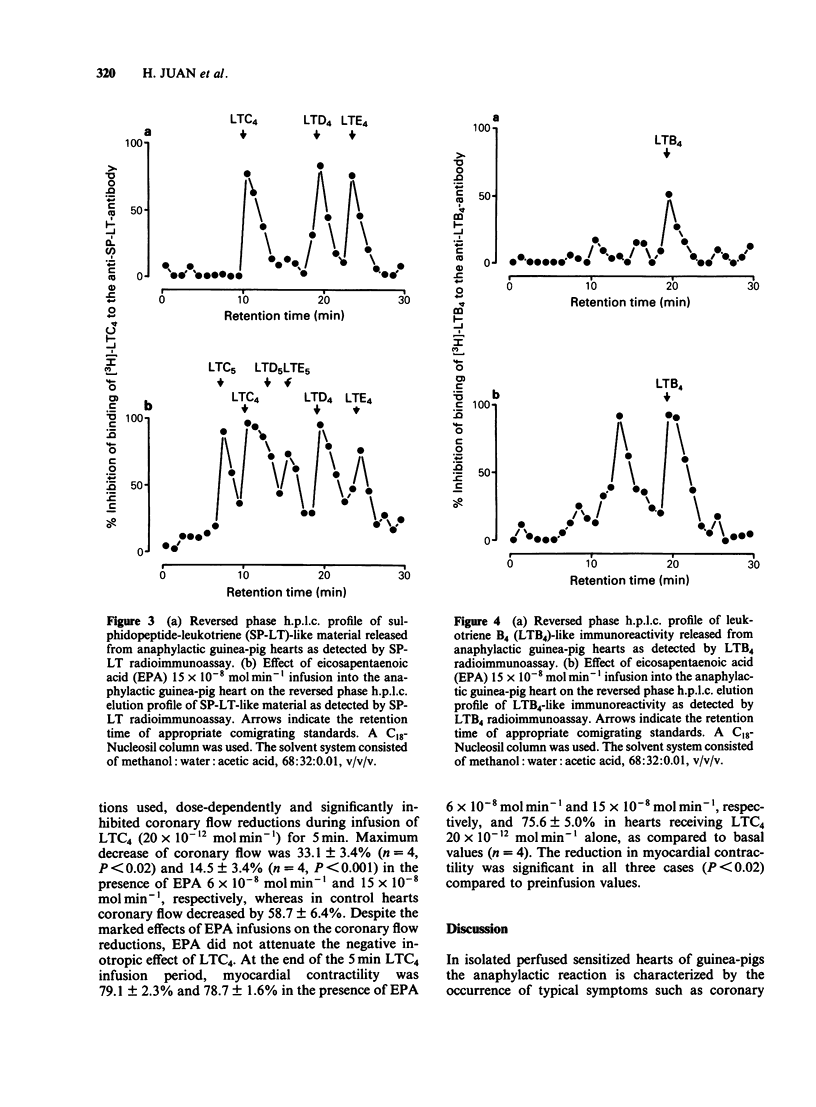
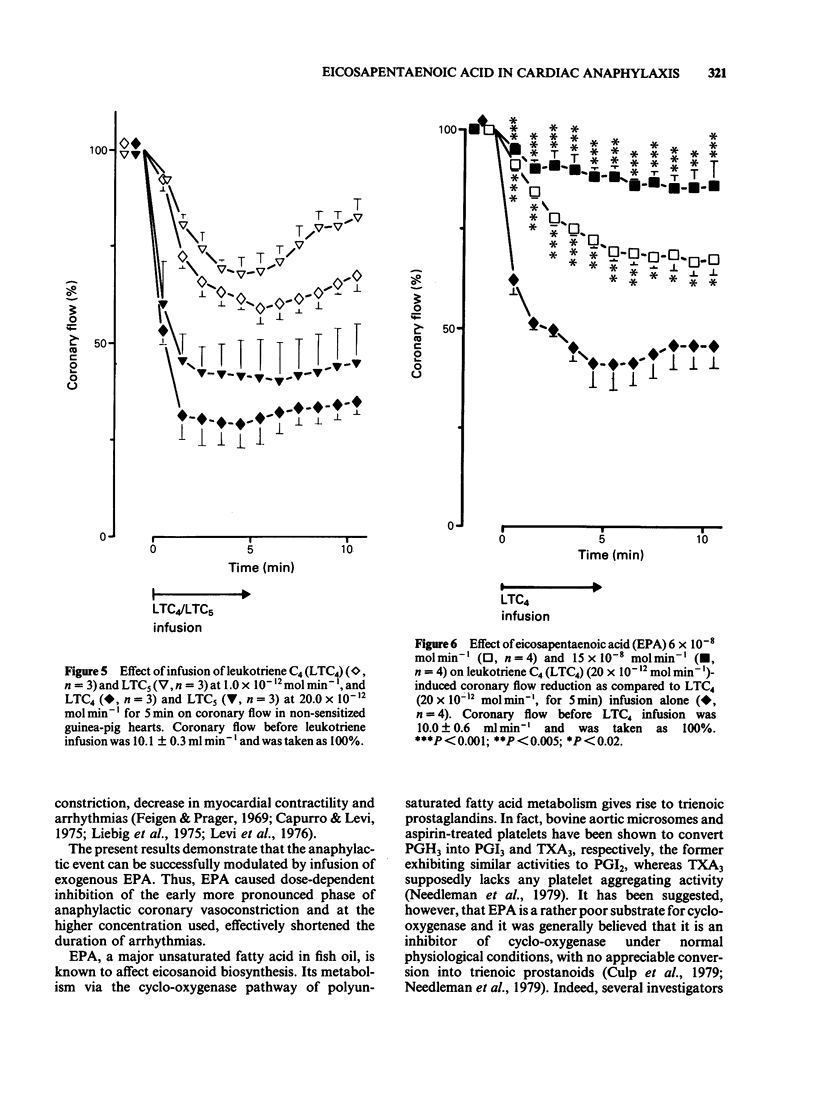
The effects of infusions of eicosapentaenoic acid (EPA) (6 X 10(-8) mol min-1 and 15 X 10(-8) mol min-1) on the coronary constriction and the release of immunoreactive sulphidopeptide-leukotrienes (SP-LT), thromboxane B2(TXB2) and 6-keto-prostaglandin F1 alpha (PGF1 alpha) from perfused anaphylactic guinea-pig hearts were investigated. EPA dose-dependently inhibited the profound early coronary flow reduction after antigen injection. The less pronounced late phase of anaphylactic coronary flow reduction was, however, not significantly affected. EPA (15 X 10(-8) mol min-1) significantly shortened the average duration of antigen-induced arrhythmias. EPA dose-dependently decreased release of immunoreactive TXB2 and 6-keto-PGF1 alpha from anaphylactic guinea-pig hearts. Release of immunoreactive SP-LT was dose-dependently increased after antigen challenge in the presence of EPA. Inhibiton of the release of SP-LT by the lipoxygenase inhibitor esculetin (1 X 10(-7) mol min-1) was accompanied by a significant attenuation of flow reduction during the late phase of anaphylactic vasoconstriction. Reversed phase h.p.l.c. of perfusates from anaphylactic guinea-pig hearts revealed immunoreactivity comigrating with authentic leukotriene C4 (LTC4), LTD4, and LTE4. In perfusates from hearts treated with EPA infusions, additional immunoreactivity was detected comigrating with LTC5, LTD5 and LTE5. In addition to immunoreactivity migrating with LTB4, as observed in control heart perfusates, in perfusates from EPA-treated hearts, a second peak was observed, which coincides with the retention time described for LTB5. Exogenous LTC5 (1 X 10(-12) mol min-1 and 20 X 10(-12) mol min-1) induced dose-dependent reductions of coronary flow and was found to be a slightly weaker constrictor than LTC4, but no significant differences were observed. Coronary vasoconstriction elicited by infusion of exogenous LTC4 (20 X 10(-12) mol min-1) was dose-dependently inhibited by infusions of EPA. However, the negative inotropic effect of LTC4 remained unaffected. Thus, in the isolated anaphylactic heart of the guinea-pig exogenous EPA was effectively metabolized via the 5-lipoxygenase pathway whereas the cyclo-oxygenase pathway of polyunsaturated fatty acid metabolism was found to be inhibited. The results are in agreement with the suggestion that cyclo-oxygenase products are mediators of the early phase of the anaphylactic coronary constriction, while vasoconstrictor SP-LT are involved in the later phase. However, in spite of enhanced release of SP-LT, EPA infusion did not result in increased coronary constriction.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aehringhaus U., Peskar B. A., Wittenberg H. R., Wölbling R. H. Effect of inhibition of synthesis and receptor antagonism of SRS-A in cardiac anaphylaxis. Br J Pharmacol. 1983 Sep;80(1):73–80. doi: 10.1111/j.1476-5381.1983.tb11051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aehringhaus U., Wölbling R. H., König W., Patrono C., Peskar B. M., Peskar B. A. Release of leukotriene C4 from human polymorphonuclear leucocytes as determined by radioimmunoassay. FEBS Lett. 1982 Sep 6;146(1):111–114. doi: 10.1016/0014-5793(82)80715-1. [DOI] [PubMed] [Google Scholar]
- Allan G., Levi R. Thromboxane and prostacyclin release during cardiac immediate hypersensitivity reactions in vitro. J Pharmacol Exp Ther. 1981 Apr;217(1):157–161. [PubMed] [Google Scholar]
- Anhut H., Bernauer W., Peskar B. A. Pharmacological modification of thromboxane and prostaglandin release in cardiac anaphylaxis. Prostaglandins. 1978 May;15(5):889–900. doi: 10.1016/0090-6980(78)90156-9. [DOI] [PubMed] [Google Scholar]
- Anhut H., Bernauer W., Peskar B. A. Radioimmunological determination of thromboxane release in cardiac anaphylaxis. Eur J Pharmacol. 1977 Jul 1;44(1):85–88. doi: 10.1016/0014-2999(77)90120-0. [DOI] [PubMed] [Google Scholar]
- BROCKLEHURST W. E. The release of histamine and formation of a slow-reacting substance (SRS-A) during anaphylactic shock. J Physiol. 1960 Jun;151:416–435. doi: 10.1113/jphysiol.1960.sp006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernström K., Orning L., Hammarström S. Gamma-Glutamyl transpeptidase, a leukotriene metabolizing enzyme. Methods Enzymol. 1982;86:38–45. doi: 10.1016/0076-6879(82)86165-x. [DOI] [PubMed] [Google Scholar]
- Burke J. A., Levi R., Guo Z. G., Corey E. J. Leukotrienes C4, D4 and E4: effects on human and guinea-pig cardiac preparations in vitro. J Pharmacol Exp Ther. 1982 Apr;221(1):235–241. [PubMed] [Google Scholar]
- Capurro N., Levi R. The heart as a target organ in systemic allergic reactions: comparison of cardiac analphylaxis in vivo and in vitro. Circ Res. 1975 Apr;36(4):520–528. doi: 10.1161/01.res.36.4.520. [DOI] [PubMed] [Google Scholar]
- Culp B. R., Titus B. G., Lands W. E. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med. 1979 Nov;3(5):269–278. doi: 10.1016/0161-4630(79)90068-5. [DOI] [PubMed] [Google Scholar]
- Dawson W., Boot J. R., Cockerill A. F., Mallen D. N., Osborne D. J. Release of novel prostaglandins and thromboxanes after immunological challenge of guinea pig lung. Nature. 1976 Aug 19;262(5570):699–702. doi: 10.1038/262699a0. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978 Jul 15;2(8081):117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Jørgensen K. A., Arnfred T. Human umbilical blood vessel converts all cis-5, 8, 11, 14, 17 eicosapentaenoic acid to prostaglandin I3. Prostaglandins. 1981 Dec;22(6):857–862. doi: 10.1016/0090-6980(81)90016-2. [DOI] [PubMed] [Google Scholar]
- Feigen G. A., Prager D. J. Experimental cardiac anaphylaxis. Physiologic, pharmacologic and biochemical aspects of immune reactions in the isolated heart. Am J Cardiol. 1969 Oct;24(4):474–491. doi: 10.1016/0002-9149(69)90490-1. [DOI] [PubMed] [Google Scholar]
- Fischer S., Weber P. C. Prostaglandin I3 is formed in vivo in man after dietary eicosapentaenoic acid. Nature. 1984 Jan 12;307(5947):165–168. doi: 10.1038/307165a0. [DOI] [PubMed] [Google Scholar]
- Fischer S., Weber P. C. Thromboxane A3 (TXA3) is formed in human platelets after dietary eicosapentaenoic acid (C20:5 omega 3). Biochem Biophys Res Commun. 1983 Nov 15;116(3):1091–1099. doi: 10.1016/s0006-291x(83)80254-x. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Korbut R., Ocetkiewicz A. Generation of prostacyclin by lungs in vivo and its release into the arterial circulation. Nature. 1978 Jun 29;273(5665):765–767. doi: 10.1038/273765a0. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Levi R. Negative inotropic effect of leukotrienes: leukotrienes C4 and D4 inhibit calcium-dependent contractile responses in potassium-depolarized guinea-pig myocardium. J Pharmacol Exp Ther. 1984 Sep;230(3):646–651. [PubMed] [Google Scholar]
- Hornstra G., Christ-Hazelhof E., Haddeman E., ten Hoor F., Nugteren D. H. Fish oil feeding lowers thromboxane- and prostacyclin production by rat platelets and aorta and does not result in the formation of prostaglandin I3. Prostaglandins. 1981 May;21(5):727–738. doi: 10.1016/0090-6980(81)90230-6. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Sams A. R., Sprecher H., Needleman P. Fatty acid structural requirements for leukotriene biosynthesis. Prostaglandins. 1980 Aug;20(2):401–410. doi: 10.1016/s0090-6980(80)80057-8. [DOI] [PubMed] [Google Scholar]
- Juan H., Sametz W. Uptake, stimulated release and metabolism of (1-14C)-eicosapentaenoic acid in a perfused organ of the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1983 Nov;324(3):207–211. doi: 10.1007/BF00503896. [DOI] [PubMed] [Google Scholar]
- Juan H., Sametz W. Vasoconstriction induced by noradrenaline and angiotensin II is antagonized by eicosapentaenoic acid independent of formation of trienoic eicosanoids. Naunyn Schmiedebergs Arch Pharmacol. 1986 Mar;332(3):288–292. doi: 10.1007/BF00504869. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Menica-Huerta J. M., Shih C., Corey E. J., Lewis R. A., Austen K. F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984 Feb 25;259(4):2383–2389. [PubMed] [Google Scholar]
- Leitch A. G., Lee T. H., Ringel E. W., Prickett J. D., Robinson D. R., Pyne S. G., Corey E. J., Drazen J. M., Austen K. F., Lewis R. A. Immunologically induced generation of tetraene and pentaene leukotrienes in the peritoneal cavities of menhaden-fed rats. J Immunol. 1984 May;132(5):2559–2565. [PubMed] [Google Scholar]
- Letts L. G., Newman D. L., Greenwald S. E., Piper P. J. Effects of intra-coronary administration of leukotriene D4 in the anaesthetized dog. Prostaglandins. 1983 Oct;26(4):563–572. doi: 10.1016/0090-6980(83)90194-6. [DOI] [PubMed] [Google Scholar]
- Letts L. G., Piper P. J. Cardiac actions of leukotrienes B4, C4, D4, and E4 in guinea pig and rat in vitro. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:391–395. [PubMed] [Google Scholar]
- Letts L. G., Piper P. J. The actions of leukotrienes C4 and D4 on guinea-pig isolated hearts. Br J Pharmacol. 1982 May;76(1):169–176. doi: 10.1111/j.1476-5381.1982.tb09203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi R., Allan G., Zavecz J. H. Prostaglandins and cardiac anaphylaxis. Life Sci. 1976 Jun 1;18(11):1255–1264. doi: 10.1016/0024-3205(76)90202-2. [DOI] [PubMed] [Google Scholar]
- Levi R., Burke J. A., Guo Z. G., Hattori Y., Hoppens C. M., McManus L. M., Hanahan D. J., Pinckard R. N. Acetyl glyceryl ether phosphorylcholine (AGEPC). A putative mediator of cardiac anaphylaxis in the guinea pig. Circ Res. 1984 Feb;54(2):117–124. doi: 10.1161/01.res.54.2.117. [DOI] [PubMed] [Google Scholar]
- Liebig R., Bernauer W., Peskar B. A. Prostaglandin, slow-reacting substance, and histamine release from anaphylactic guinea-pig hearts, and its pharmacological modification. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(1):65–76. doi: 10.1007/BF00498030. [DOI] [PubMed] [Google Scholar]
- Michelassi F., Landa L., Hill R. D., Lowenstein E., Watkins W. D., Petkau A. J., Zapol W. M. Leukotriene D4: a potent coronary artery vasoconstrictor associated with impaired ventricular contraction. Science. 1982 Aug 27;217(4562):841–843. doi: 10.1126/science.6808665. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Piper P. J., Tippins J. R. Slow-reacting substance of anaphylaxis: studies on purification and characterisation. Agents Actions Suppl. 1979;(6):27–36. doi: 10.1007/978-3-0348-7232-4_3. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neichi T., Koshihara Y., Murota S. Inhibitory effect of esculetin on 5-lipoxygenase and leukotriene biosynthesis. Biochim Biophys Acta. 1983 Aug 29;753(1):130–132. [PubMed] [Google Scholar]
- Peskar B. M., Kozuschek W., Morgenroth K., Hoppe U., Dreyling K. W., Peskar B. A. Leukotriensynthese durch gastrointestinales Gewebe und ihre pharmakologische Beeinflussung. Wien Klin Wochenschr. 1986 Feb 21;98(4):98–104. [PubMed] [Google Scholar]
- Piper P. J., Tippins J. R., Morris H. R., Taylor G. W. Potentiation of SRS-A release from guinea pig chopped lung by substrates for arachidonate lipoxygenase. Adv Prostaglandin Thromboxane Res. 1980;6:121–124. [PubMed] [Google Scholar]
- Roth D. M., Lefer D. J., Hock C. E., Lefer A. M. Effects of peptide leukotrienes on cardiac dynamics in rat, cat, and guinea pig hearts. Am J Physiol. 1985 Sep;249(3 Pt 2):H477–H484. doi: 10.1152/ajpheart.1985.249.3.H477. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Schrör K., Moncada S., Ubatuba F. B., Vane J. R. Transformation of arachidonic acid and prostaglandin endoperoxides by the guinea pig heart. Formation of RCS and prostacyclin. Eur J Pharmacol. 1978 Jan 1;47(1):103–114. doi: 10.1016/0014-2999(78)90380-1. [DOI] [PubMed] [Google Scholar]
- Sekiya K., Okuda H., Arichi S. Selective inhibition of platelet lipoxygenase by esculetin. Biochim Biophys Acta. 1982 Oct 14;713(1):68–72. [PubMed] [Google Scholar]
- Simmet T., Peskar B. A. Eicosanoids and the coronary circulation. Rev Physiol Biochem Pharmacol. 1986;104:1–64. doi: 10.1007/BFb0031012. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Kaduce T. L., Figard P. H., Norton K. C., Hoak J. C., Czervionke R. L. Eicosapentaenoic acid and prostacyclin production by cultured human endothelial cells. J Lipid Res. 1983 Dec;24(12):1595–1604. [PubMed] [Google Scholar]
- Strasser T., Fischer S., Weber P. C. Leukotriene B5 is formed in human neutrophils after dietary supplementation with icosapentaenoic acid. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1540–1543. doi: 10.1073/pnas.82.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano T., Salmon J. A., Moncada S. Biosynthesis and biological activity of leukotriene B5. Prostaglandins. 1984 Feb;27(2):217–232. doi: 10.1016/0090-6980(84)90075-3. [DOI] [PubMed] [Google Scholar]
- Terashita Z. I., Fukui H., Nishikawa K., Hirata M., Kikuchi S. Coronary vasospastic action of thromboxane A2 in isolated, working guinea pig hearts. Eur J Pharmacol. 1978 Dec 15;53(1):49–56. doi: 10.1016/0014-2999(78)90266-2. [DOI] [PubMed] [Google Scholar]


