Abstract
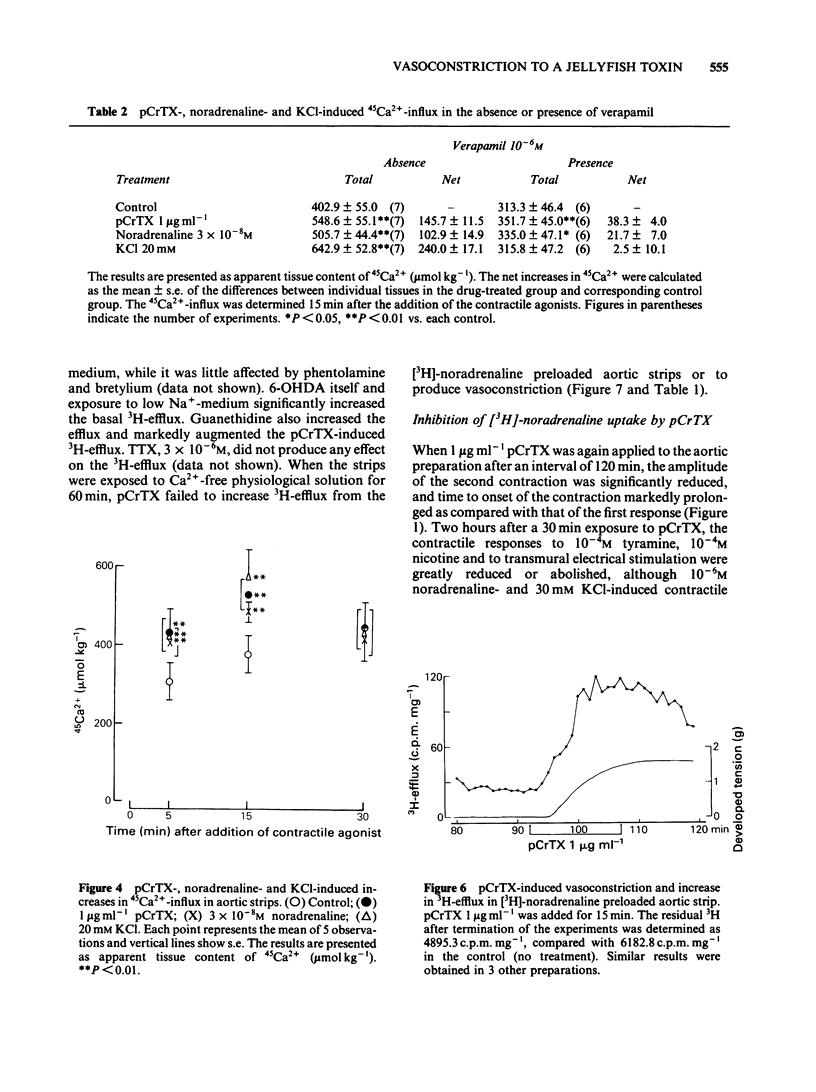
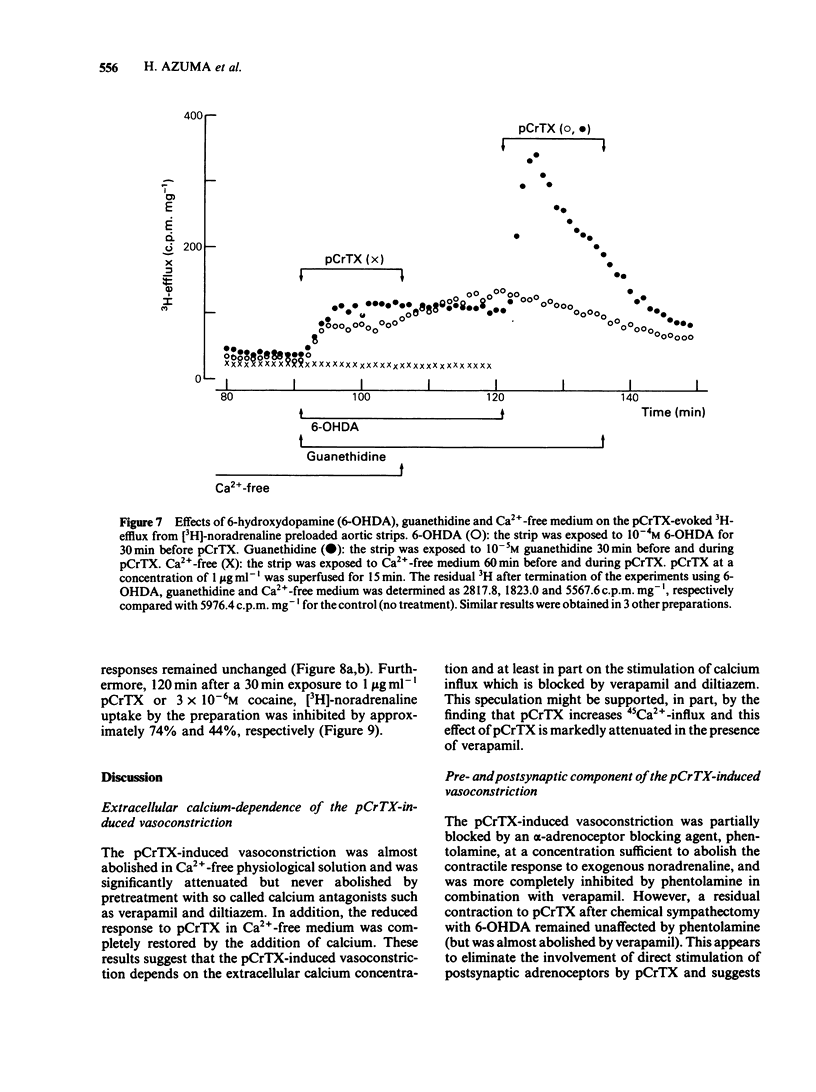
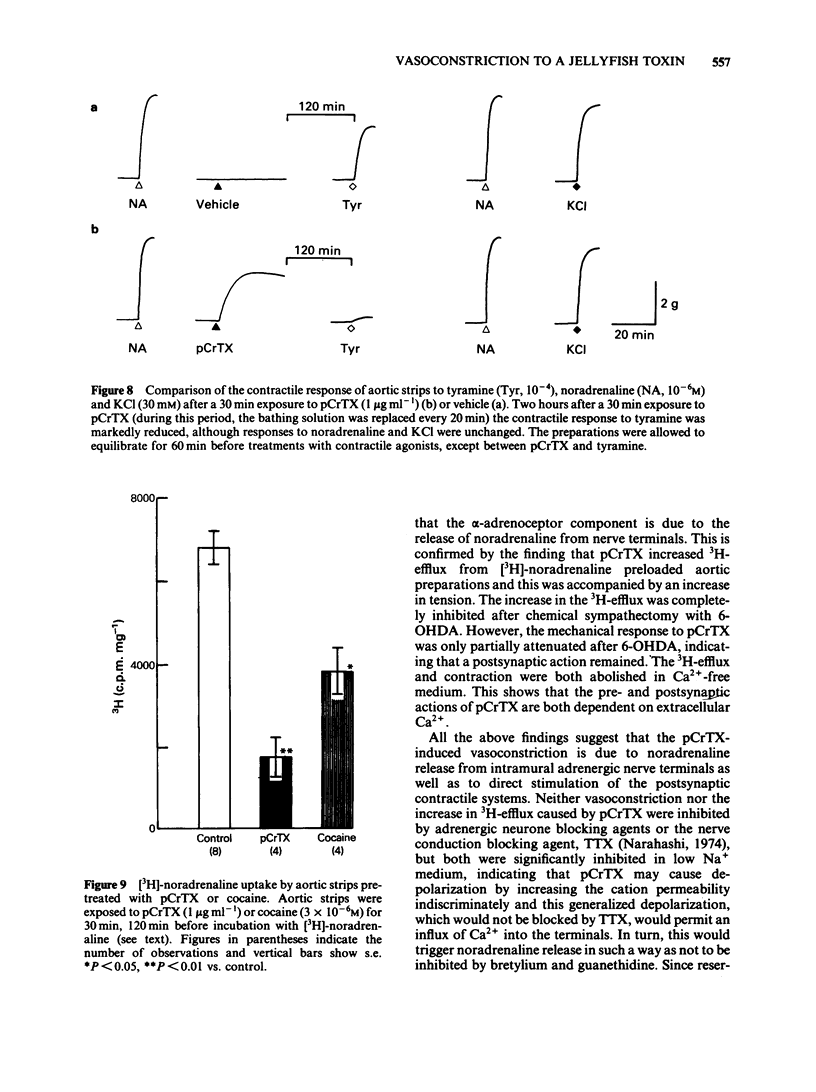
The purpose of the present experiments was to investigate the pharmacological mechanisms of the vasoconstriction caused by the toxin (pCrTX) which had been partially purified from the tentacles of the jellyfish Carybdea rastonii ('Andonkurage'). pCrTX (0.1 to 10 micrograms ml-1) produced a tonic contraction of rabbit aortic strips, which was nearly abolished in Ca2+-free medium and was significantly reduced by verapamil or diltiazem. pCrTX stimulated 45Ca2+-influx and this effect was markedly attenuated by verapamil. pCrTX-induced vasoconstriction was significantly attenuated by phentolamine, 6-hydroxydopamine (6-OHDA) and in low Na+-medium, but not by bretylium, guanethidine, reserpinization or tetrodotoxin (TTX). pCrTX continuously and significantly increased the 3H-efflux from [3H]-noradrenaline preloaded aortic strips and this effect was completely inhibited by pretreatment with 6-OHDA and in Ca2+-free medium, but not by phentolamine, bretylium, guanethidine or TTX. A single exposure to pCrTX for 30 min greatly reduced the contractile responses to tyramine, nicotine and transmural electrical stimulation, but not those to noradrenaline or KC1. In addition, incorporation of [3H]-noradrenaline was reduced. Pretreatments with chlorphenylamine or indomethacin failed to modify the contractile response to pCrTX. These results suggest that the pCrTX-induced vasoconstriction is caused by a presynaptic action, releasing noradrenaline from the intramural adrenergic nerve terminals, and by a postsynaptic action, which consists at least in part of stimulation of the transmembrane calcium influx. Both pre- and postsynaptic actions depend on the external calcium concentration. The data further suggest that pCrTX damages the noradrenaline uptake and/or storage mechanisms without damaging postsynaptic contractile systems.
Full text
PDF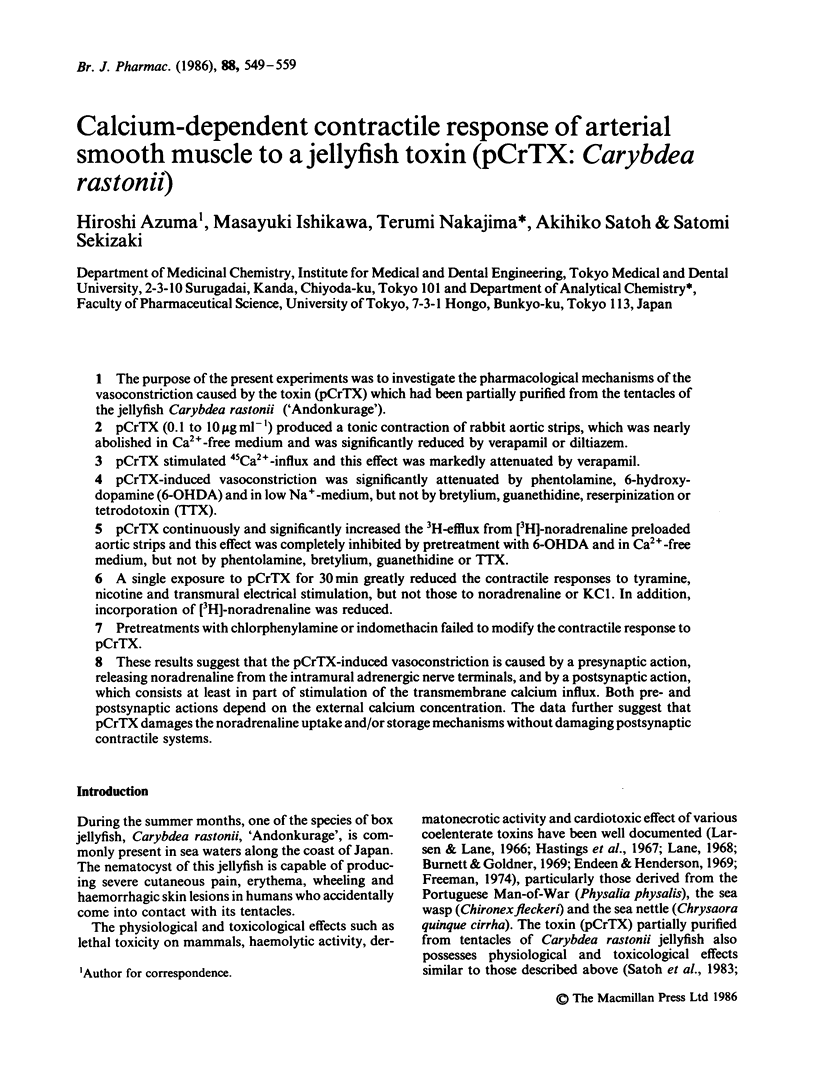




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berner P. F., Disalvo J., Schwartz A. Differential inhibitory effects of the ionophore RO2-2985 (X537A) on contractile responses to potassium and histamine in coronary artery smooth muscle. J Pharmacol Exp Ther. 1980 Apr;213(1):59–63. [PubMed] [Google Scholar]
- Burnett J. W., Goldner R. Effects of Chrysaora quinquecirrha (sea nettle) toxin on the rat cardiovascular system. Proc Soc Exp Biol Med. 1969 Oct;132(1):353–356. doi: 10.3181/00379727-132-34213. [DOI] [PubMed] [Google Scholar]
- Endean R., Henderson L. Further studies of toxic material from nematocysts of the cubomedusan Chironex fleckeri Southcott. Toxicon. 1969 Dec;7(4):303–314. doi: 10.1016/0041-0101(69)90030-0. [DOI] [PubMed] [Google Scholar]
- Freeman S. E. Actions of Chironex fleckeri toxins on cardiac transmembrane potentials. Toxicon. 1974 Aug;12(4):395–404. doi: 10.1016/0041-0101(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Godfraind T. Calcium exchange in vascular smooth muscle, action of noradrenaline and lanthanum. J Physiol. 1976 Aug;260(1):21–35. doi: 10.1113/jphysiol.1976.sp011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., Dieu D. The inhibition by flunarizine of the norepinephrine-evoked contraction and calcium influx in rat aorta and mesenteric arteries. J Pharmacol Exp Ther. 1981 May;217(2):510–515. [PubMed] [Google Scholar]
- Hastings S. G., Larsen J. B., Lane C. E. Effects of nematocyst toxin of Physalia physalis (Portuguese Man-of-War) on the canine cardiovascular system. Proc Soc Exp Biol Med. 1967 May;125(1):41–45. doi: 10.3181/00379727-125-32008. [DOI] [PubMed] [Google Scholar]
- Hudgins P. M., Harris T. M. Further studies on the effects of reserpine pretreatment on rabbit aorta: calcium and histologic changes. J Pharmacol Exp Ther. 1970 Dec;175(3):609–618. [PubMed] [Google Scholar]
- Jonsson G., Sachs C. Uptake and accumulation of 3 H-6-hydroxydopamine in adrenergic nerves. Eur J Pharmacol. 1971 Sep;16(1):55–62. doi: 10.1016/0014-2999(71)90056-2. [DOI] [PubMed] [Google Scholar]
- Juan H. Role of calcium in prostaglandin E release induced by bradykinin and the ionophore A 23187. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jun;307(2):177–183. doi: 10.1007/BF00498461. [DOI] [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Effects of calcium antagonists on release of [3H]noradrenaline in rabbit aorta. Eur J Pharmacol. 1984 Jun 1;101(3-4):177–183. doi: 10.1016/0014-2999(84)90154-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen J. B., Lane C. E. Some effects of Physalia physalis toxin on the cardiovascular system of the rat. Toxicon. 1966 Nov;4(3):199–203. doi: 10.1016/0041-0101(66)90050-x. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Pinto J. E., Trifaró J. M. The different effects of D-600 (methoxyverapamil) on the release of adrenal catecholamines induced by acetylcholine, high potassium or sodium deprivation. Br J Pharmacol. 1976 May;57(1):127–132. doi: 10.1111/j.1476-5381.1976.tb07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Bevan J. A. The release of H3-norepinephrine in arterial strips studied by the technique of superfusion and transmural stimulation. J Pharmacol Exp Ther. 1970 Mar;172(1):62–68. [PubMed] [Google Scholar]
- Vanhoutte P. M., Verbeuren T. J., Webb R. C. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981 Jan;61(1):151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]


