Abstract
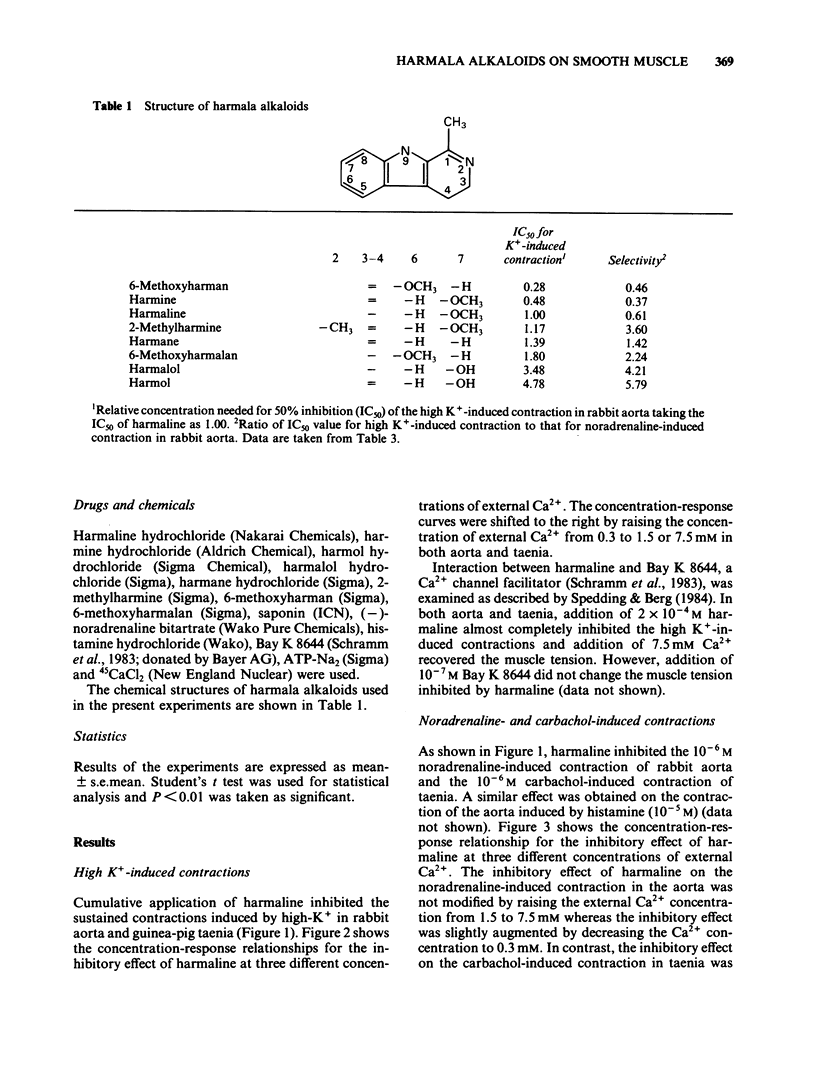
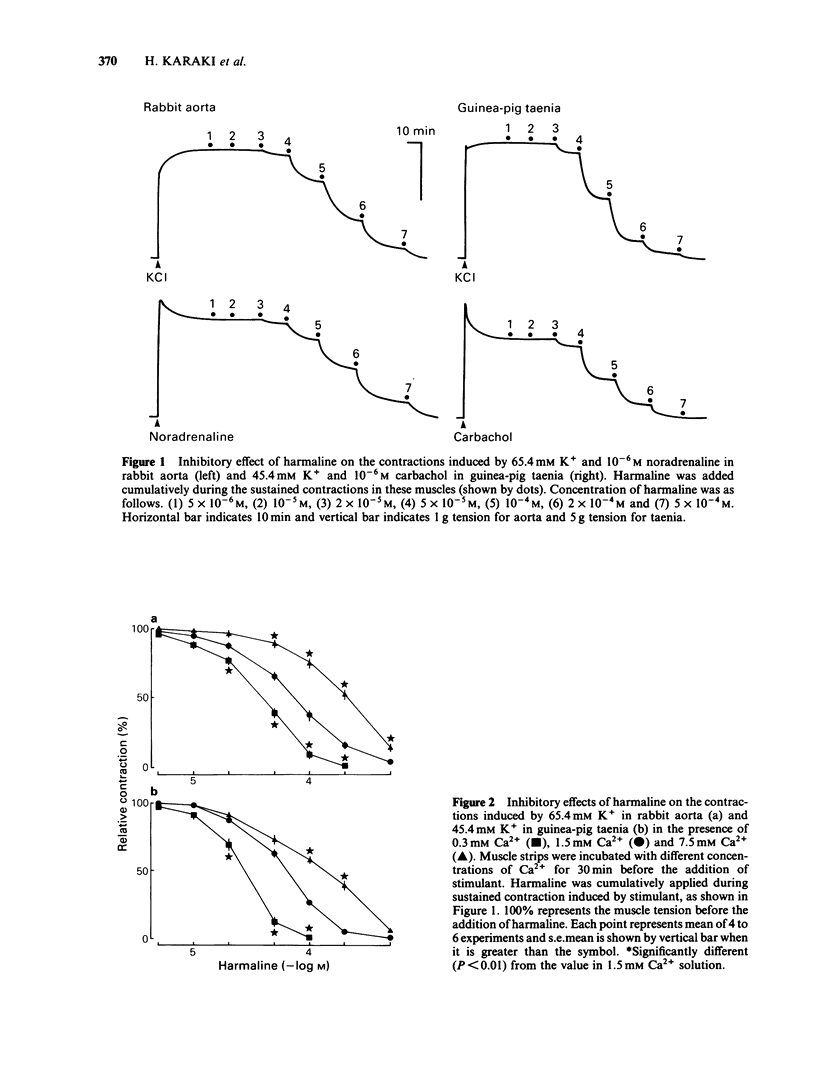
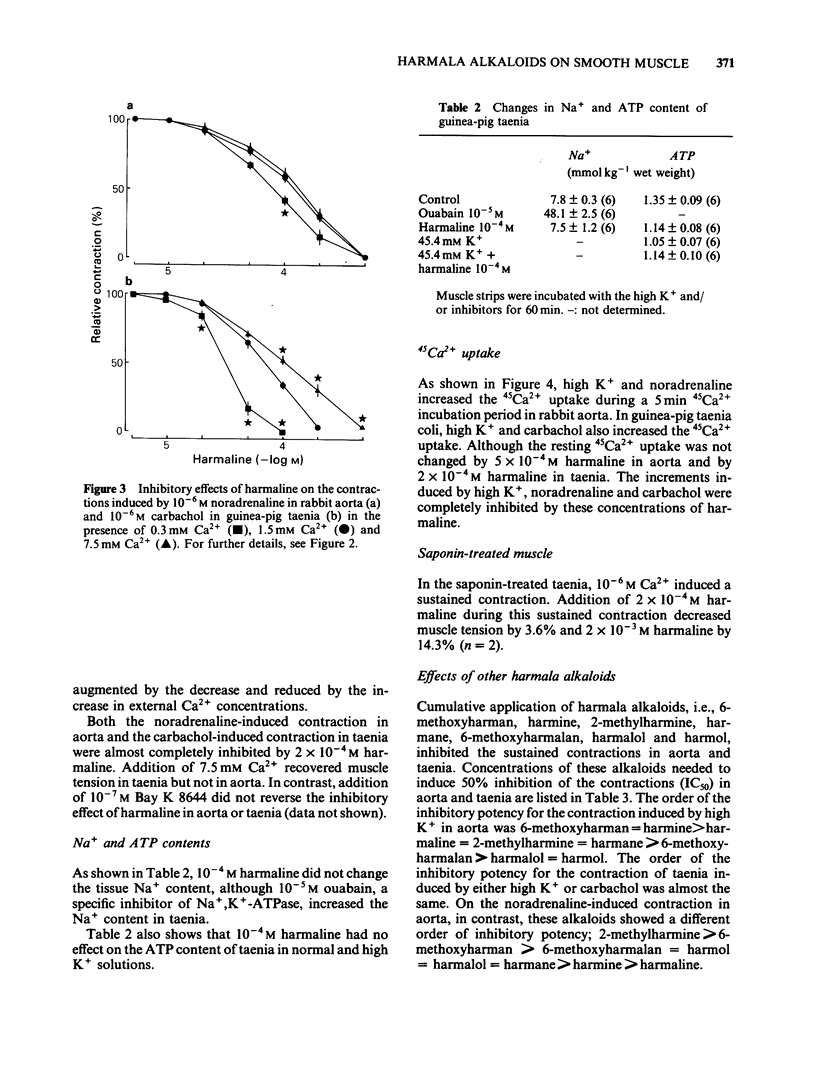
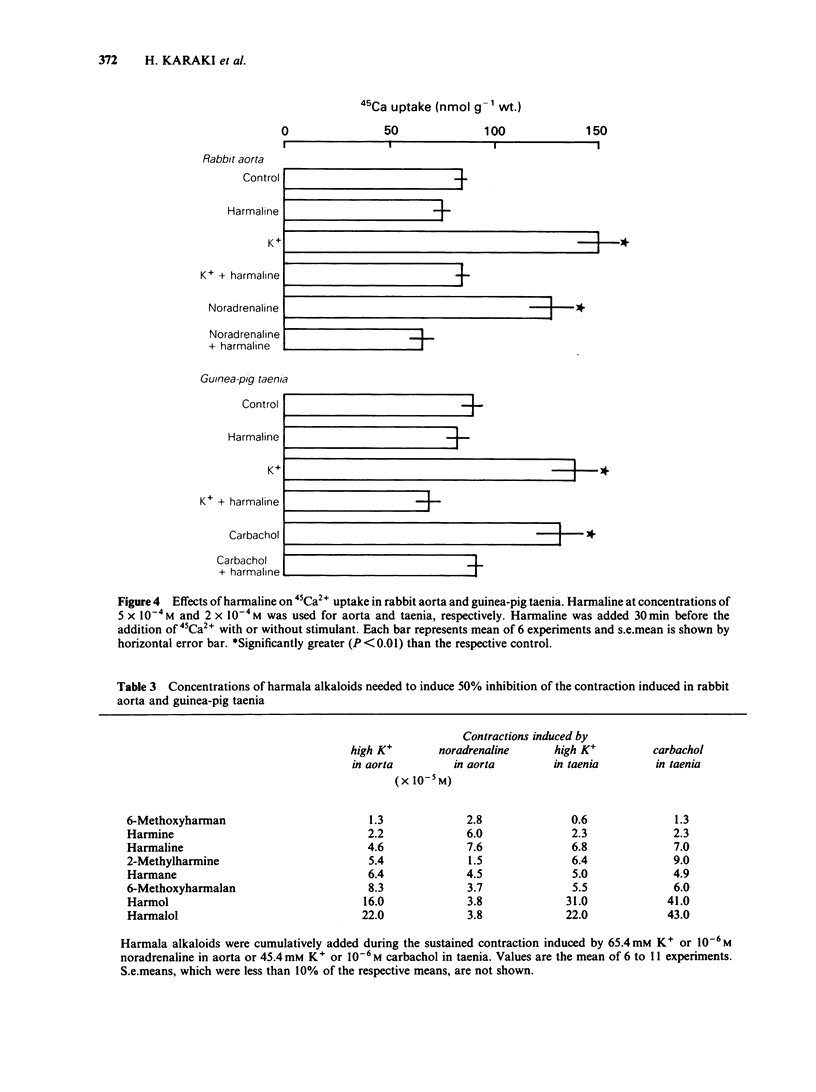
Effects of harmaline and other harmala alkaloids on the contractions induced in the vascular smooth muscle of rabbit aorta and intestinal smooth muscle of taenia isolated from guinea-pig caecum were examined. In rabbit isolated aorta, harmaline inhibited the sustained contraction induced by 65.4 mM K+ with an IC50 (concentration needed for 50% inhibition) of 4.6 X 10(-5) M. This inhibitory effect on high K+-induced contraction was antagonized by raising the concentration of external Ca2+ but not by Bay K 8644, a Ca2+ channel facilitator. Harmaline also inhibited the sustained contraction induced by noradrenaline (10(-6) M) with an IC50 of 7.6 X 10(-5) M. The inhibitory effects on noradrenaline-induced contractions were not antagonized by raising the external Ca2+ concentrations or by Bay K 8644. In guinea-pig taenia, harmaline inhibited the 45.4 mM K+-induced contraction with an IC50 of 6.8 X 10(-5) M and the carbachol (10(-6) M)-induced contraction with an IC50 of 7.0 X 10(-5) M. The inhibitory effects on both high K+- and carbachol-induced contractions were antagonized by raising the external Ca2+ concentrations but not by Bay K 8644. Harmaline, at the concentrations needed to inhibit the muscle contraction, inhibited the increase in 45Ca2+ uptake induced by high K+, noradrenaline and carbachol in aorta and taenia. Harmaline did not change the cellular Na+ and ATP contents in resting and high K+ stimulated taenia. Other harmala alkaloids also inhibited the contractions in these smooth muscles.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. H., Willis J. S. The effect of harmaline on unidirectional potassium fluxes and ouabain binding in renal cell cultures. Biochim Biophys Acta. 1983 Jan 5;727(1):144–150. doi: 10.1016/0005-2736(83)90378-4. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bose D. Mechanism of mechanical inhibition of smooth muscle by ouabain. Br J Pharmacol. 1975 Sep;55(1):111–116. doi: 10.1111/j.1476-5381.1975.tb07618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa M., Jaimovich E., de la Fuente M. Harmaline: a competitive inhibitor of Na ion in the (Na+ + K+)-ATPase system. J Membr Biol. 1973 Oct 10;13(3):263–282. doi: 10.1007/BF01868232. [DOI] [PubMed] [Google Scholar]
- Cauvin C., Loutzenhiser R., Van Breemen C. Mechanisms of calcium antagonist-induced vasodilation. Annu Rev Pharmacol Toxicol. 1983;23:373–396. doi: 10.1146/annurev.pa.23.040183.002105. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Smart L., Suleiman M. S. The effect of harmaline and related beta-carbolines on the acetylcholine-stimulated contractions of guinea-pig ileum. Eur J Pharmacol. 1981 Apr 9;70(4):429–436. doi: 10.1016/0014-2999(81)90353-8. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Smart L., Suleiman M. S. The effect of harmaline and related harmala alkaloids on ouabain-stimulated contractions of the guinea-pig ileum. Eur J Pharmacol. 1981 Apr 24;71(1):87–92. doi: 10.1016/0014-2999(81)90389-7. [DOI] [PubMed] [Google Scholar]
- Karaki H., Kubota H., Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979 Jun 15;56(3):237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Comparative effects of verapamil and sodium nitroprusside on contraction and 45Ca uptake in the smooth muscle of rabbit aorta, rat aorta and guinea-pig taenia coli. Br J Pharmacol. 1984 Feb;81(2):393–400. doi: 10.1111/j.1476-5381.1984.tb10091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Effects of calcium antagonists on release of [3H]noradrenaline in rabbit aorta. Eur J Pharmacol. 1984 Jun 1;101(3-4):177–183. doi: 10.1016/0014-2999(84)90154-7. [DOI] [PubMed] [Google Scholar]
- Karaki H., Suzuki T., Ozaki H., Urakawa N., Ishida Y. Dissociation of K+-induced tension and cellular Ca2+ retention in vascular and intestinal smooth muscle in normoxia and hypoxia. Pflugers Arch. 1982 Aug;394(2):118–123. doi: 10.1007/BF00582912. [DOI] [PubMed] [Google Scholar]
- Karaki H., Urakawa N. Possible role of endogenous catecholamines in the contractions induced in rabbit aorta by ouabain, sodium depletion and potassium depletion. Eur J Pharmacol. 1977 May 1;43(1):65–72. doi: 10.1016/0014-2999(77)90161-3. [DOI] [PubMed] [Google Scholar]
- Karaki H., Weiss G. B. Alterations in high and low affinity binding of 45Ca in rabbit aortic smooth muscle by norepinephrine and potassium after exposure to lanthanum and low temperature. J Pharmacol Exp Ther. 1979 Oct;211(1):86–92. [PubMed] [Google Scholar]
- Karaki H., Weiss G. B. Calcium channels in smooth muscle. Gastroenterology. 1984 Oct;87(4):960–970. [PubMed] [Google Scholar]
- Kishimoto T., Ozaki H., Urakawa N. A quantitative relationship between cellular Na accumulation and relaxation produced by ouabain in the depolarized smooth muscle of guinea-pig taenia coli. Naunyn Schmiedebergs Arch Pharmacol. 1980 Jun;312(2):199–207. doi: 10.1007/BF00569731. [DOI] [PubMed] [Google Scholar]
- Oashi H., Takewaki T., Okada T. Calcium and the contractile effect of carbachol in the depolarized guinea-pig taenia caecum. Jpn J Pharmacol. 1974 Aug;24(4):601–611. doi: 10.1254/jjp.24.601. [DOI] [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Schultes R. E. Hallucinogens of plant origin. Science. 1969 Jan 17;163(3864):245–254. doi: 10.1126/science.163.3864.245. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Buclon M., Robinson J. W. L'harmaline, un inhibiteur des processus de transport intestinal associés aux mouvements des ions sodium. Gastroenterol Clin Biol. 1977;1(1):87–93. [PubMed] [Google Scholar]
- Spedding M., Berg C. Interactions between a "calcium channel agonist", Bay K 8644, and calcium antagonists differentiate calcium antagonist subgroups in K+-depolarized smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1984 Nov;328(1):69–75. doi: 10.1007/BF00496109. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Karaki H., Urakawa N. Mechanism of inhibition of contraction by high K, Na deficient solution in smooth muscle of guinea-pig taenia coli. Arch Int Pharmacodyn Ther. 1980 Nov;248(1):43–49. [PubMed] [Google Scholar]
- Van Breemen C., Aaronson P., Loutzenhiser R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978 Jun;30(2):167–208. [PubMed] [Google Scholar]


