Abstract
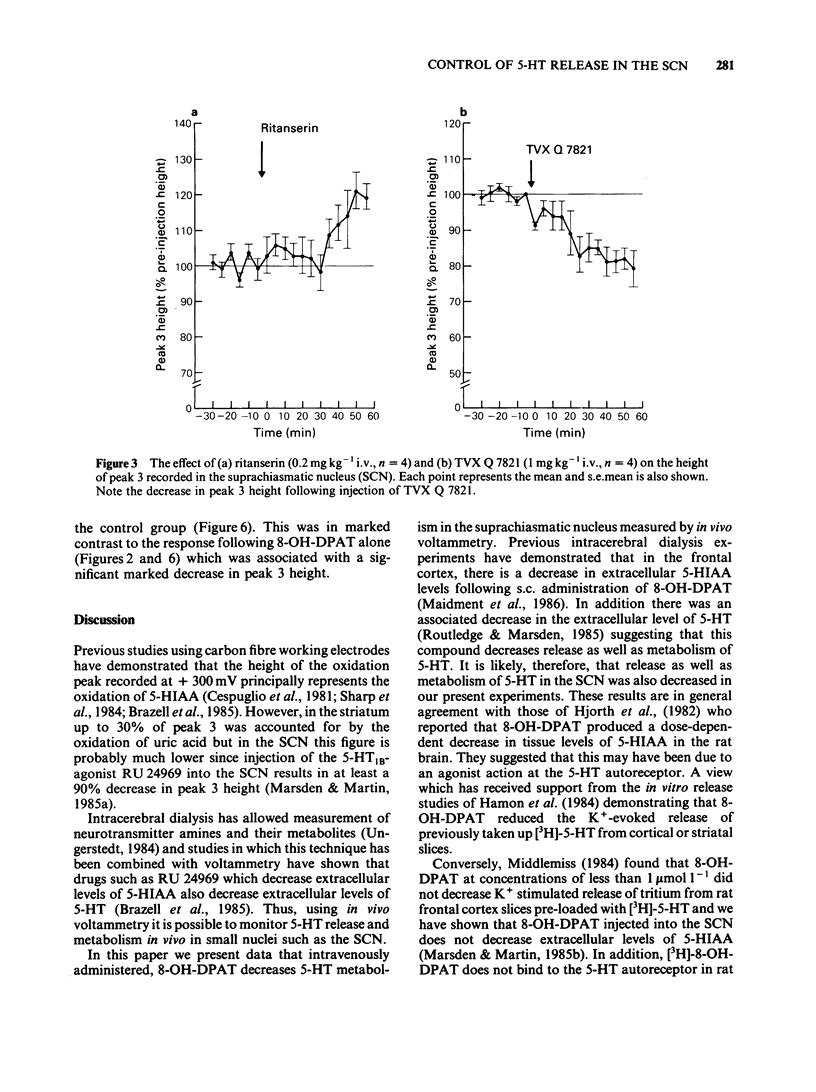
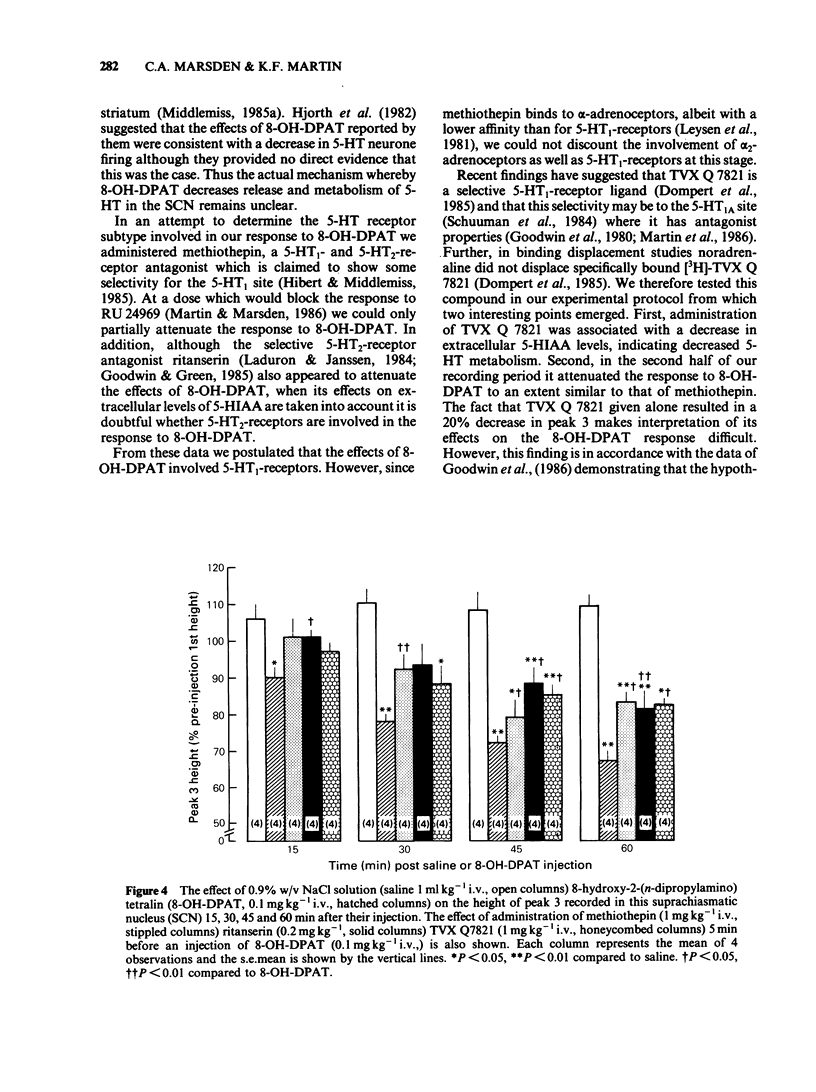
The 5-hydroxytryptamine1A-receptor agonist 8-hydroxy-2-(n-dipropylamino) tetralin (8-OH-DPAT, 0.1 mg kg-1 i.v.) decreased the height of the extracellular 5-hydroxyindoleacetic acid (5-HIAA) oxidation peak recorded in the suprachiasmatic nucleus (SCN) of the anaesthetized rat by use of differential pulse voltammetry. The decrease in extracellular 5-HIAA produced by 8-OH-DPAT could be partially attenuated by prior administration of the non-selective 5-HT receptor antagonist methiothepin (1 mg kg-1 i.v.). The 5-HT2-receptor antagonist ritanserin (0.2 mg kg-1 i.v.) did not appear to block the effects of 8-OH-DPAT. The selective ligand for 5-HT1A recognition sites TVX Q 7821 (isapirone, 1 mg kg-1 i.v.) decreased the extracellular level of 5-HIAA in the SCN but to a lesser extent than 8-OH-DPAT. The response to 8-OH-DPAT was attenuated by prior administration of TVX Q 7821 to a level suggesting that TVX Q 7821 had blocked the effect of intravenous 8-OH-DPAT. Idazoxan (0.2 mg kg-1 i.v.) an alpha 2-adrenoceptor antagonist, completely blocked the effect of 8-OH-DPAT on the 5-HIAA oxidation peak recorded in the SCN, whilst having no effect on the 5-HIAA oxidation peak when given alone. At a dose of 0.5 mg kg-1 i.v. idazoxan induced a 120% increase in the height of the indole oxidation peak, suggesting that 5-HT release and metabolism in the rat SCN may be influenced by tonic adrenergic inputs. The data in this paper suggest that 5-HT1A- and alpha 2-receptors are involved in the effects of i.v. administered 8-OH-DPAT on 5-HT release and metabolism in the SCN in vivo.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P. A., Waldmeier P. C. Negative feedback control of serotonin release in vivo: comparison of 5-hydroxyindolacetic acid levels measured by voltammetry in conscious rats and by biochemical techniques. Neuroscience. 1984 Jan;11(1):195–204. doi: 10.1016/0306-4522(84)90223-9. [DOI] [PubMed] [Google Scholar]
- Brazell M. P., Marsden C. A., Nisbet A. P., Routledge C. The 5-HT1 receptor agonist RU-24969 decreases 5-hydroxytryptamine (5-HT) release and metabolism in the rat frontal cortex in vitro and in vivo. Br J Pharmacol. 1985 Sep;86(1):209–216. doi: 10.1111/j.1476-5381.1985.tb09451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespuglio R., Faradji H., Ponchon J. L., Buda M., Riou F., Gonon F., Pujol J. F., Jouvet M. Differential pulse voltammetry in brain tissue. I. Detection of 5-hydroxyindoles in the rat striatum. Brain Res. 1981 Nov 2;223(2):287–298. doi: 10.1016/0006-8993(81)91142-2. [DOI] [PubMed] [Google Scholar]
- Crespi F., Sharp T., Maidment N., Marsden C. Differential pulse voltammetry in vivo--evidence that uric acid contributes to the indole oxidation peak. Neurosci Lett. 1983 Dec 30;43(2-3):203–207. doi: 10.1016/0304-3940(83)90188-x. [DOI] [PubMed] [Google Scholar]
- Dompert W. U., Glaser T., Traber J. 3H-TVX Q 7821: identification of 5-HT1 binding sites as target for a novel putative anxiolytic. Naunyn Schmiedebergs Arch Pharmacol. 1985 Feb;328(4):467–470. doi: 10.1007/BF00692918. [DOI] [PubMed] [Google Scholar]
- Doods H. N., Kalkman H. O., De Jonge A., Thoolen M. J., Wilffert B., Timmermans P. B., Van Zwieten P. A. Differential selectivities of RU 24969 and 8-OH-DPAT for the purported 5-HT1A and 5-HT1B binding sites. Correlation between 5-HT1A affinity and hypotensive activity. Eur J Pharmacol. 1985 Jun 19;112(3):363–370. doi: 10.1016/0014-2999(85)90782-4. [DOI] [PubMed] [Google Scholar]
- Doxey J. C., Roach A. G., Smith C. F. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983 Mar;78(3):489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel G., Göthert M., Hoyer D., Schlicker E., Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Faradji H., Cespuglio R., Jouvet M. Voltammetric measurements of 5-hydroxyindole compounds in the suprachiasmatic nuclei: circadian fluctuations. Brain Res. 1983 Nov 21;279(1-2):111–119. doi: 10.1016/0006-8993(83)90168-3. [DOI] [PubMed] [Google Scholar]
- Galzin A. M., Moret C., Langer S. Z. Evidence that exogenous but not endogenous norepinephrine activates the presynaptic alpha-2 adrenoceptors on serotonergic nerve endings in the rat hypothalamus. J Pharmacol Exp Ther. 1984 Mar;228(3):725–732. [PubMed] [Google Scholar]
- Goodwin G. M., Green A. R. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br J Pharmacol. 1985 Mar;84(3):743–753. doi: 10.1111/j.1476-5381.1985.tb16157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göthert M., Huth H. Alpha-adrenoceptor-mediated modulation of 5-hydroxytryptamine release from rat brain cortex slices. Naunyn Schmiedebergs Arch Pharmacol. 1980 Aug;313(1):21–26. doi: 10.1007/BF00505800. [DOI] [PubMed] [Google Scholar]
- Göthert M., Huth H., Schlicker E. Characterization of the receptor subtype involved in alpha-adrenoceptor-mediated modulation of serotonin release from rat brain cortex slices. Naunyn Schmiedebergs Arch Pharmacol. 1981 Nov;317(3):199–203. doi: 10.1007/BF00503816. [DOI] [PubMed] [Google Scholar]
- Hamon M., Bourgoin S., Gozlan H., Hall M. D., Goetz C., Artaud F., Horn A. S. Biochemical evidence for the 5-HT agonist properties of PAT (8-hydroxy-2-(di-n-propylamino)tetralin) in the rat brain. Eur J Pharmacol. 1984 May 4;100(3-4):263–276. doi: 10.1016/0014-2999(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Awouters F., Kennis L., Laduron P. M., Vandenberk J., Janssen P. A. Receptor binding profile of R 41 468, a novel antagonist at 5-HT2 receptors. Life Sci. 1981 Mar 2;28(9):1015–1022. doi: 10.1016/0024-3205(81)90747-5. [DOI] [PubMed] [Google Scholar]
- Maidment N. T., Marsden C. A. In vivo voltammetric and behavioural evidence for somatodendritic autoreceptor control of mesolimbic dopamine neurones. Brain Res. 1985 Jul 15;338(2):317–325. doi: 10.1016/0006-8993(85)90162-3. [DOI] [PubMed] [Google Scholar]
- Martin K. F., Marsden C. A. In vivo voltammetry in the suprachiasmatic nucleus of the rat: effects of RU24969, methiothepin and ketanserin. Eur J Pharmacol. 1986 Feb 11;121(1):135–139. doi: 10.1016/0014-2999(86)90403-6. [DOI] [PubMed] [Google Scholar]
- Martin L. L., Sanders-Bush E. Comparison of the pharmacological characteristics of 5 HT1 and 5 HT2 binding sites with those of serotonin autoreceptors which modulate serotonin release. Naunyn Schmiedebergs Arch Pharmacol. 1982 Dec;321(3):165–170. doi: 10.1007/BF00505480. [DOI] [PubMed] [Google Scholar]
- Middlemiss D. N. 8-Hydroxy-2-(di-n-propylamino) tetralin is devoid of activity at the 5-hydroxytryptamine autoreceptor in rat brain. Implications for the proposed link between the autoreceptor and the [3H] 5-HT recognition site. Naunyn Schmiedebergs Arch Pharmacol. 1984 Aug;327(1):18–22. doi: 10.1007/BF00504986. [DOI] [PubMed] [Google Scholar]
- Middlemiss D. N., Fozard J. R. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol. 1983 May 20;90(1):151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Middlemiss D. N. The putative 5-HT1 receptor agonist, RU 24969, inhibits the efflux of 5-hydroxytryptamine from rat frontal cortex slices by stimulation of the 5-HT autoreceptor. J Pharm Pharmacol. 1985 Jun;37(6):434–437. doi: 10.1111/j.2042-7158.1985.tb03032.x. [DOI] [PubMed] [Google Scholar]
- Pedigo N. W., Yamamura H. I., Nelson D. L. Discrimination of multiple [3H]5-hydroxytryptamine binding sites by the neuroleptic spiperone in rat brain. J Neurochem. 1981 Jan;36(1):220–226. doi: 10.1111/j.1471-4159.1981.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979 Nov;16(3):687–699. [PubMed] [Google Scholar]
- Ponchon J. L., Cespuglio R., Gonon F., Jouvet M., Pujol J. F. Normal pulse polarography with carbon fiber electrodes for in vitro and in vivo determination of catecholamines. Anal Chem. 1979 Aug;51(9):1483–1486. doi: 10.1021/ac50045a030. [DOI] [PubMed] [Google Scholar]
- Routledge C., Marsden C. A. Recent advances in high-performance liquid chromatographic analysis of small molecules. Application of high-performance liquid chromatography with electrochemical detection to the study of neurotransmitters in vivo. Biochem Soc Trans. 1985 Dec;13(6):1058–1061. doi: 10.1042/bst0131058. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G., Waters S. J., Nelson D. L. [3H]5-Hydroxytryptamine binding sites: species and tissue variation. J Neurochem. 1984 Jan;42(1):65–70. doi: 10.1111/j.1471-4159.1984.tb09699.x. [DOI] [PubMed] [Google Scholar]
- Sills M. A., Wolfe B. B., Frazer A. Determination of selective and nonselective compounds for the 5-HT 1A and 5-HT 1B receptor subtypes in rat frontal cortex. J Pharmacol Exp Ther. 1984 Dec;231(3):480–487. [PubMed] [Google Scholar]



