Abstract
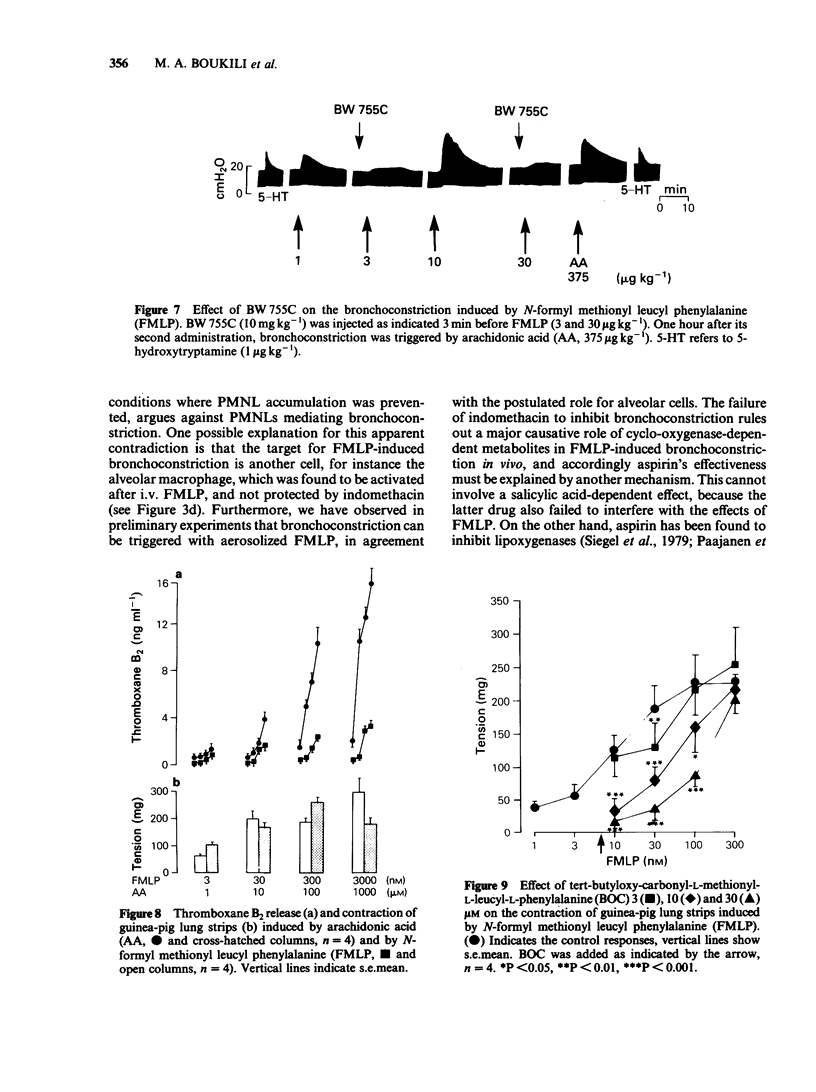
The intravenous administration of the chemotactic and secretagogue peptide N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP; 0.3-30 micrograms kg-1) to the guinea-pig induces bronchoconstriction and dose-dependent leukopenia accompanied by mild thrombocytopenia. No electron microscopic evidence of platelet aggregation in lungs or significant accumulation of 111In-labelled platelets in the thoracic region at the height of bronchoconstriction was noted. Bronchoconstriction and leukopenia induced by FMLP were not affected by prostacyclin, by platelet depletion, by the platelet-activating factor (Paf-acether) antagonist BN 52021 or by the histamine H1-antagonist mepyramine. Bronchoconstriction, but not leukopenia, was inhibited by aspirin, whereas the peptido-leukotriene antagonist compound FPL 55712 and the cyclo-oxygenase lipoxygenase inhibitor indomethacin reduced bronchoconstriction to a limited extent only. The mixed cyclo-oxygenase/lipoxygenase inhibitor compound BW 755C was very effective in blocking bronchoconstriction by the highest dose of FMLP used, but failed to interfere with leukopenia. FMLP-induced dose-dependent contraction of parenchymal lung strips was accompanied by the formation of immuno-reactive thromboxane B2 in amounts markedly less than those formed from exogenous arachidonic acid at concentrations equieffective in inducing contractions. FMLP-induced contractions of the guinea-pig lung strip were not modified by mepyramine nor by FPL 55712. They were reduced by indomethacin and aspirin and an even greater reduction was obtained with aspirin used in combination with FPL 55712. BW 755C suppressed the effects of all the concentrations of FMLP tested, whereas tert-butyloxy-carbonyl-L-methionyl-L-leucyl-L-phenylalanine, a chemical analogue of FMLP, displaced the concentration-response curve to the right, without reducing the maximal contraction obtained. The present results indicate that: (a) bronchoconstriction by FMLP is not due to platelet activation, to cyclo-oxygenase-dependent mechanisms or to peptido-leukotriene formation. The inhibitory effect of aspirin and BW 755C involves a property other than cyclo-oxygenase inhibition, which is not shared by indomethacin. (b) The contractile effects of FMLP on parenchymal lung strips follow an interaction with specific receptor sites, as shown by the effectiveness of tert-butyloxy-carbonyl-L-methionyl-L-leucyl-L-phenylalanine, and involves the combined effects of cyclo-oxygenase and lipoxygenase metabolites.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. H., O'Donnell M., Simko B. A., Welton A. F. An in vivo model for measuring antigen-induced SRS-A-mediated bronchoconstriction and plasma SRS-A levels in the guinea-pig. Br J Pharmacol. 1983 Jan;78(1):67–74. doi: 10.1111/j.1476-5381.1983.tb09363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augstein J., Farmer J. B., Lee T. B., Sheard P., Tattersall M. L. Selective inhibitor of slow reacting substance of anaphylaxis. Nat New Biol. 1973 Oct 17;245(146):215–217. doi: 10.1038/newbio245215a0. [DOI] [PubMed] [Google Scholar]
- Dahinden C., Fehr J. Receptor-directed inhibition of chemotactic factor-induced neutrophil hyperactivity by pyrazolon derivatives. Definition of a chemotactic peptide antagonist. J Clin Invest. 1980 Nov;66(5):884–891. doi: 10.1172/JCI109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham S. R., Carroll M., Walsh G. M., Kay A. B. Leukocyte activation in allergen-induced late-phase asthmatic reactions. N Engl J Med. 1984 Nov 29;311(22):1398–1402. doi: 10.1056/NEJM198411293112202. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort J., Vargaftig B. B. Role of platelets in aspirin-sensitive bronchoconstriction in the guinea-pig; interactions with salicylic acid. Br J Pharmacol. 1978 May;63(1):35–42. doi: 10.1111/j.1476-5381.1978.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellouch-Tubiana A., Lefort J., Pirotzky E., Vargaftig B. B., Pfister A. Ultrastructural evidence for extravascular platelet recruitment in the lung upon intravenous injection of platelet-activating factor (PAF-acether) to guinea-pigs. Br J Exp Pathol. 1985 Jun;66(3):345–355. [PMC free article] [PubMed] [Google Scholar]
- Nagy L., Lee T. H., Kay A. B. Neutrophil chemotactic activity in antigen-induced late asthmatic reactions. N Engl J Med. 1982 Mar 4;306(9):497–501. doi: 10.1056/NEJM198203043060901. [DOI] [PubMed] [Google Scholar]
- Paajanen H., Männistö J., Uotila P. Aspirin inhibits arachidonic acid metabolism via lipoxygenase and cyclo-oxygenase in hamster isolated lungs. Prostaglandins. 1982 May;23(5):731–741. doi: 10.1016/s0090-6980(82)80011-7. [DOI] [PubMed] [Google Scholar]
- Page C. P., Paul W., Morley J. An in vivo model for studying platelet aggregation and disaggregation. Thromb Haemost. 1982 Jun 28;47(3):210–213. [PubMed] [Google Scholar]
- Page C. P., Paul W., Morley J. Platelets and bronchospasm. Int Arch Allergy Appl Immunol. 1984;74(4):347–350. doi: 10.1159/000233571. [DOI] [PubMed] [Google Scholar]
- Pham Huy D., Roch-Arveiller M., Muntaner O., Giroud J. P. Effect of some anti-inflammatory drugs on FMLP-induced chemotaxis and random migration of rat polymorphonuclear leucocytes. Eur J Pharmacol. 1985 May 8;111(2):251–256. doi: 10.1016/0014-2999(85)90764-2. [DOI] [PubMed] [Google Scholar]
- Pretolani M., Page C. P., Lefort J., Lagente V., Vargaftig B. B. Pharmacological modulation of the respiratory and haematological changes accompanying active anaphylaxis in the guinea-pig. Eur J Pharmacol. 1986 Jun 24;125(3):403–409. doi: 10.1016/0014-2999(86)90796-x. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Schulz R., Seeger W. Release of leukotrienes into the perfusate of calcium-ionophore stimulated rabbit lungs. Influence of 5-lipoxygenase inhibitors. Biochem Pharmacol. 1986 Jan 15;35(2):183–193. doi: 10.1016/0006-2952(86)90512-5. [DOI] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Cuatrecasas P. Aspirin-like drugs interfere with arachidonate metabolism by inhibition of the 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid peroxidase activity of the lipoxygenase pathway. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3774–3778. doi: 10.1073/pnas.76.8.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sors H., Pradelles P., Dray F., Rigaud M., Maclouf J., Bernard P. Analytical methods for thromboxane B2 measurement and validation of radioimmunoassay by gas liquid chromatography-mass spectrometry. Prostaglandins. 1978 Aug;16(2):277–290. doi: 10.1016/0090-6980(78)90030-8. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Schultz E. L., Albertine K. H. Leucocytes and pulmonary microvascular injury. Ann N Y Acad Sci. 1982;384:332–343. doi: 10.1111/j.1749-6632.1982.tb21382.x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Dao N. Release of vasoactive substances from guinea-pig lungs by slow-reacting substance c and arachidonic acid. Its blockade by nonsteroid anti-inflammatory agents. Pharmacology. 1971;6(2):99–108. doi: 10.1159/000136231. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J. Differential effects of prostacyclin and prostaglandin E1 on bronchoconstriction and thrombocytopenia during collagen and arachidonate infusions and anaphylactic shock in the guinea-pig. Prostaglandins. 1979 Oct;18(4):519–528. doi: 10.1016/0090-6980(79)90020-0. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Joseph D., Fouque F. Mechanisms of bronchoconstriction and of thrombocytopenia induced by collagen in the guinea pig. Eur J Pharmacol. 1979 Oct 1;58(3):273–284. doi: 10.1016/0014-2999(79)90476-x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Murphy R. C. Inhibition by aspirin of bronchoconstriction due to leukotrienes C4 and D4 in the guinea pig. Eur J Pharmacol. 1981 Jul 10;72(4):417–418. doi: 10.1016/0014-2999(81)90589-6. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Wal F., Chignard M. Role of the metabolites of arachidonate in platelet-dependent and independent experimental bronchoconstriction. Bull Eur Physiopathol Respir. 1981;17(4):723–736. [PubMed] [Google Scholar]