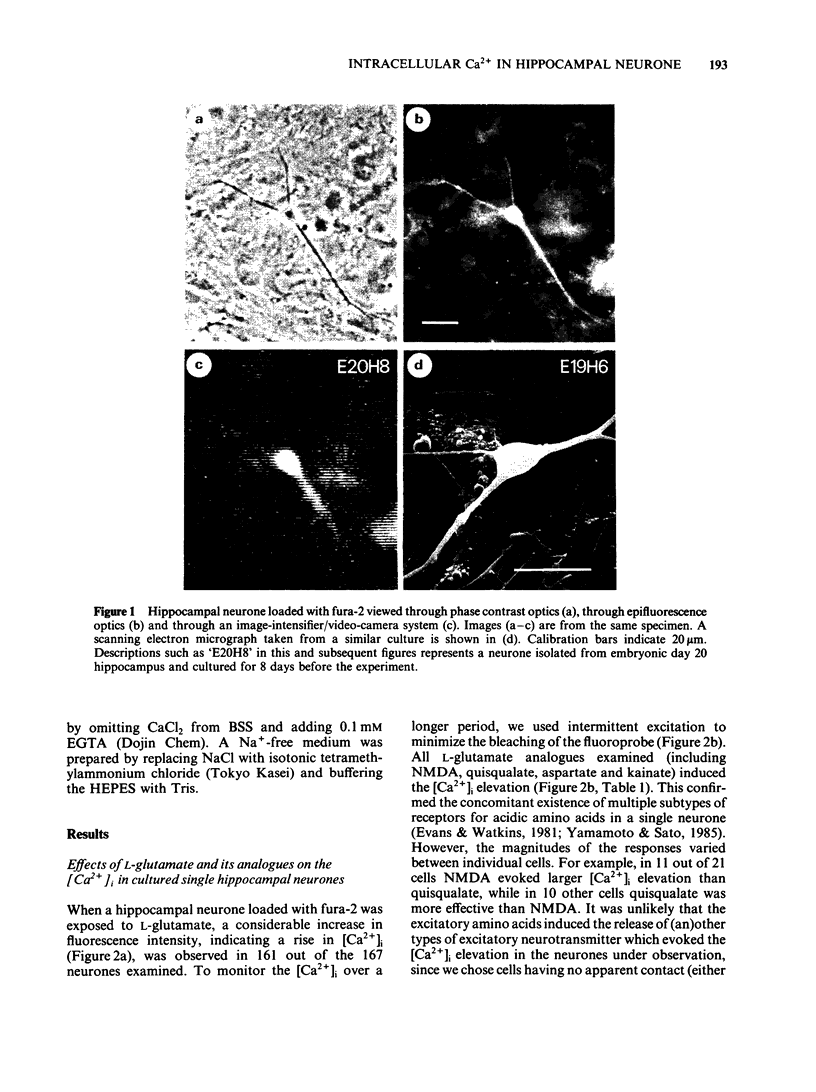
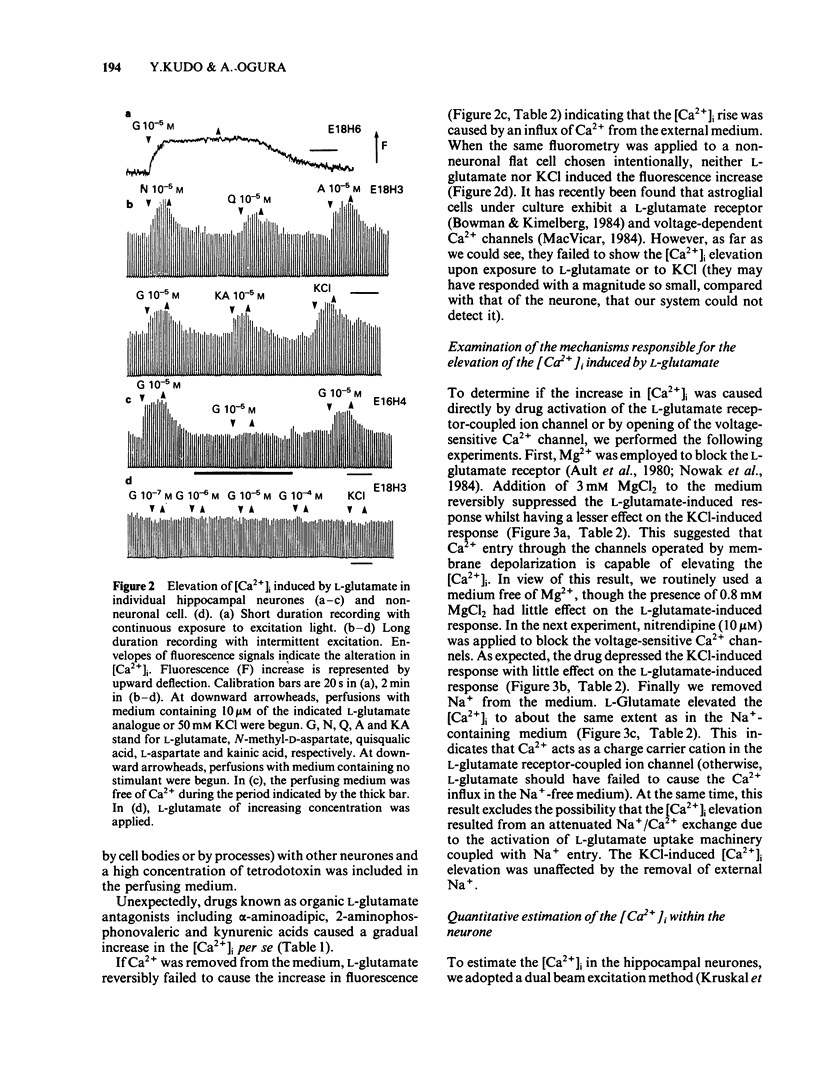
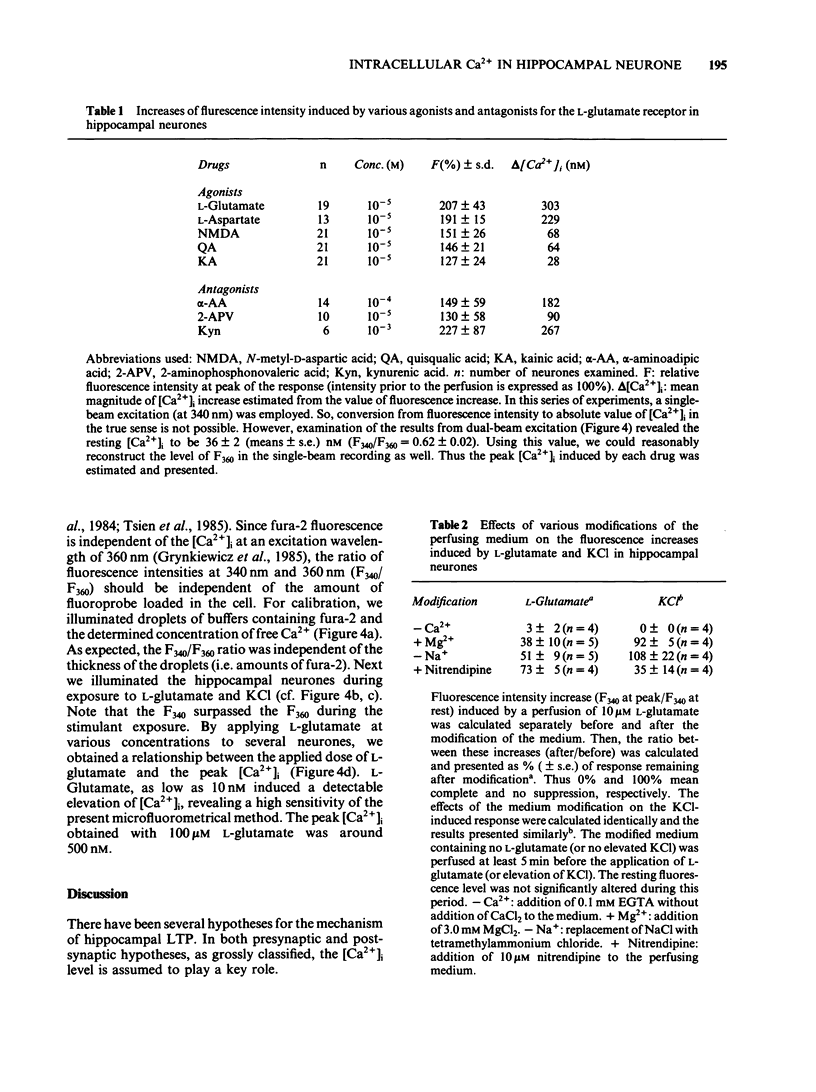
Abstract
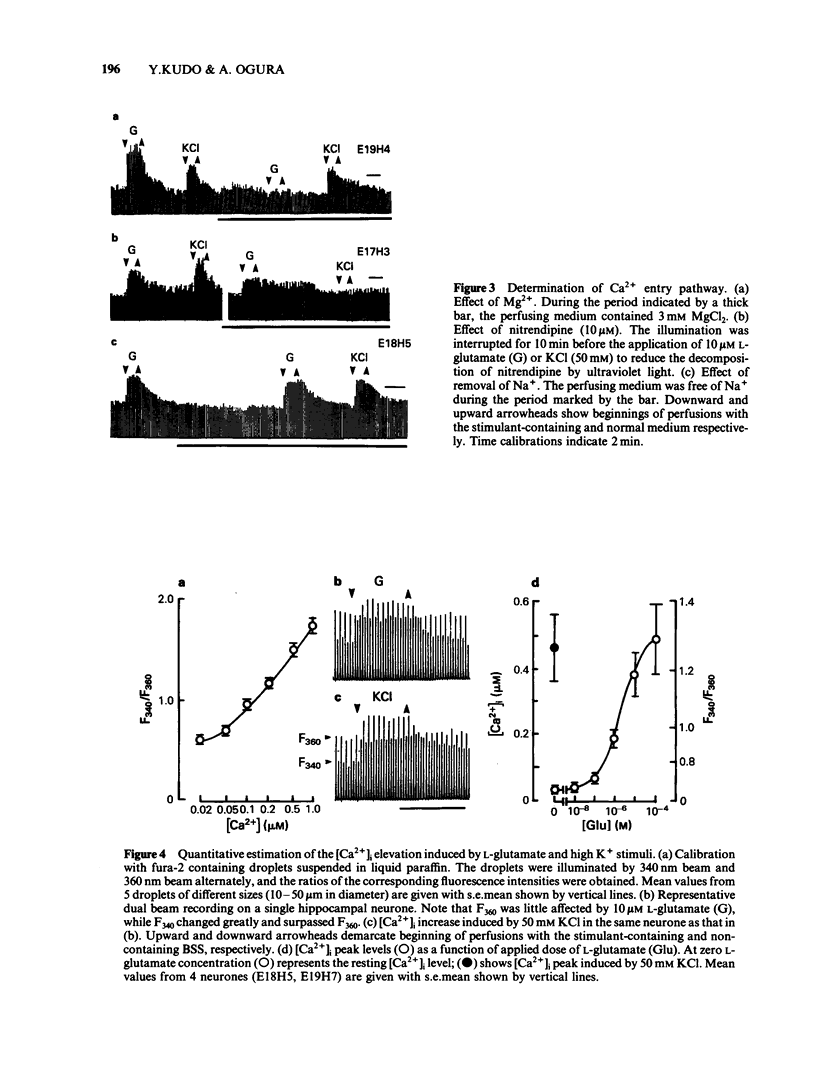
A system for real-time quantitative monitoring of intracellular free calcium ion concentration ([Ca2+]i) on a single cell basis was developed by the combination of a fluorescent Ca2+ indicator fura-2, a fluorescence microscope, a video-camera and photometrical devices. It was applied to rat individual hippocampal neurones under culture for detection of L-glutamate-induced alterations in the [Ca2+]i level. L-Glutamate (0.01-100 microM) induced a dose-dependent elevation of the [Ca2+]i. The [Ca2+]i in the rat hippocampal neurone was found to be around 30 nM in the resting state, and was increased up to 500 nM by the application of 100 microM L-glutamate. N-methyl-D-aspartate, kainate and quisqualate in a concentration of 10 microM also increased the [Ca2+]i level in the same single neurone, but their efficacy varied between individual cells. The L-glutamate-induced [Ca2+]i elevation was abolished after removal of extracellular Ca2+ and was much reduced by Mg2+ (3 mM). The increase was, however, still observed in a Na+-free medium. The L-glutamate-induced [Ca2+]i elevation was not affected substantially after treatment with nitrendipine (10 microM) which blocked the increase in [Ca2+]i induced by an isotonic high KCl-medium (50 mM). The present results suggest that the L-glutamate-induced [Ca2+]i elevation in the hippocampal neurone is due to an influx of Ca2+ through both L-glutamate receptor-coupled and voltage-sensitive ionic channels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Baudry M., Lynch G. Regulation of hippocampal glutamate receptors: evidence for the involvement of a calcium-activated protease. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2298–2302. doi: 10.1073/pnas.77.4.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. L., Kimelberg H. K. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984 Oct 18;311(5987):656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983 Jan;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Hamon B., Konnerth A. GABA and baclofen reduce changes in extracellular free calcium in area CA1 of rat hippocampal slices. Neurosci Lett. 1984 Jun 29;47(3):295–300. doi: 10.1016/0304-3940(84)90529-9. [DOI] [PubMed] [Google Scholar]
- Kruskal B. A., Keith C. H., Maxfield F. R. Thyrotropin-releasing hormone-induced changes in intracellular [Ca2+] measured by microspectrofluorometry on individual quin2-loaded cells. J Cell Biol. 1984 Sep;99(3):1167–1172. doi: 10.1083/jcb.99.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Lynch M. A., Errington M. L., Bliss T. V. Long-term potentiation of synaptic transmission in the dentate gyrus: increased release of [14C]glutamate without increase in receptor binding. Neurosci Lett. 1985 Nov 20;62(1):123–129. doi: 10.1016/0304-3940(85)90295-2. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A. Voltage-dependent calcium channels in glial cells. Science. 1984 Dec 14;226(4680):1345–1347. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Dinerstein R. J., Miller R. J. Depolarization-induced increases in intracellular free calcium detected in single cultured neuronal cells. Neurosci Lett. 1984 Oct 12;51(2):165–170. doi: 10.1016/0304-3940(84)90545-7. [DOI] [PubMed] [Google Scholar]
- Siman R., Baudry M., Lynch G. Regulation of glutamate receptor binding by the cytoskeletal protein fodrin. Nature. 1985 Jan 17;313(5999):225–228. doi: 10.1038/313225a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Sato H. Some properties of ionic channels activated by excitatory amino acids in hippocampal neurons. Exp Brain Res. 1985;57(2):313–320. doi: 10.1007/BF00236537. [DOI] [PubMed] [Google Scholar]