Abstract
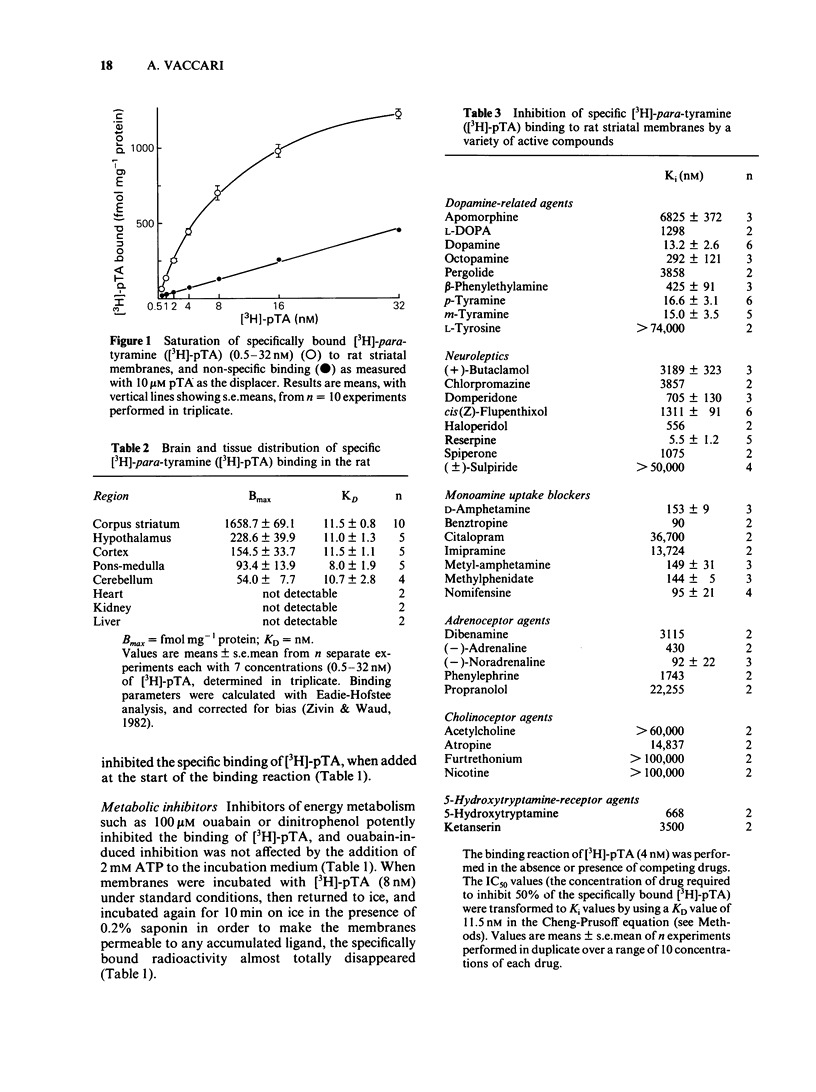
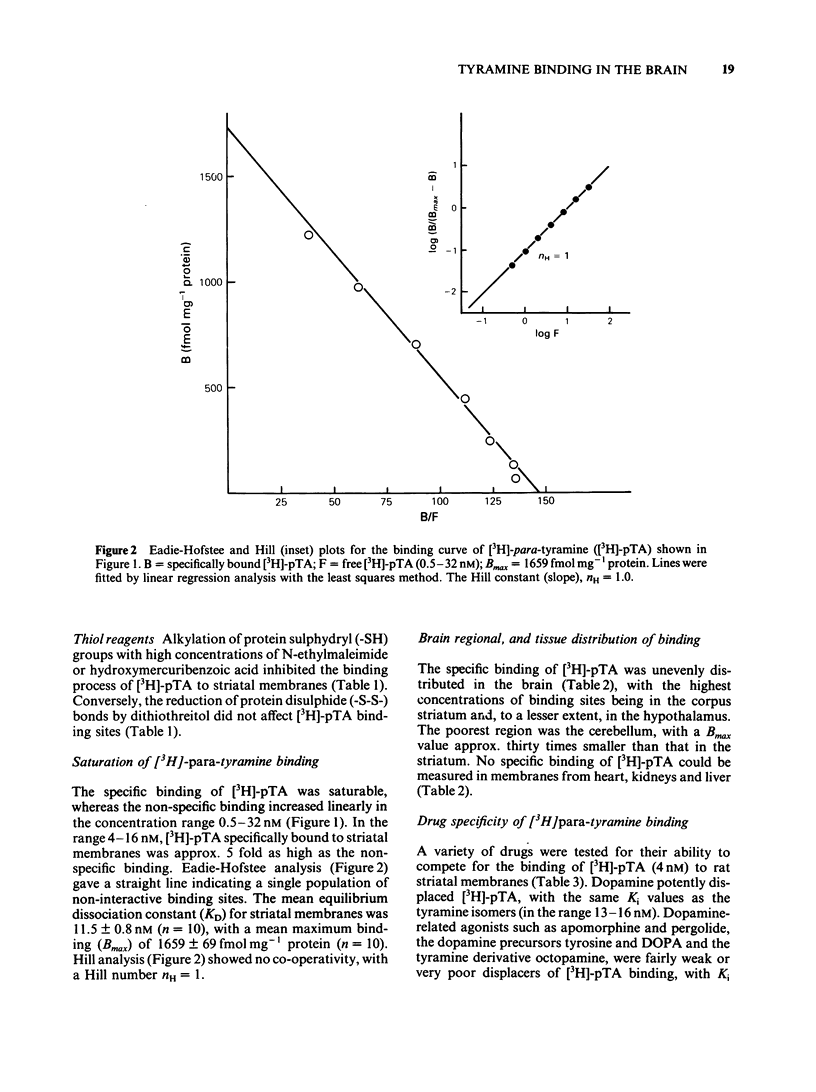
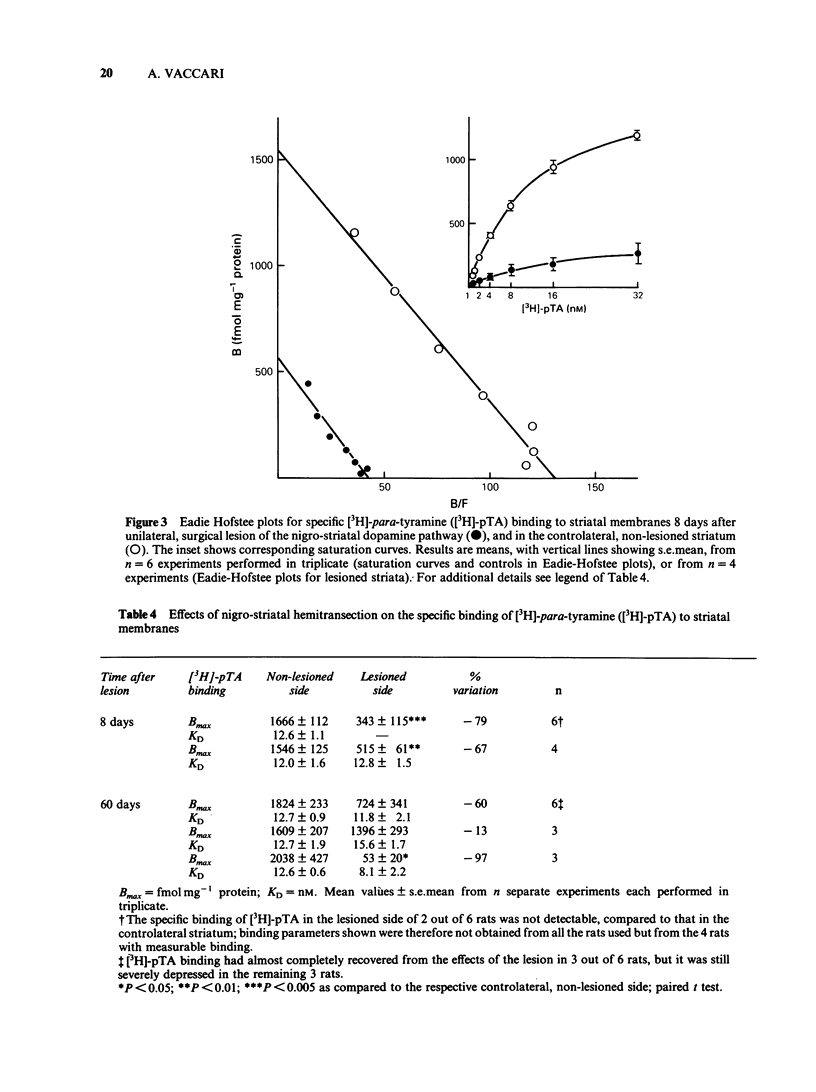
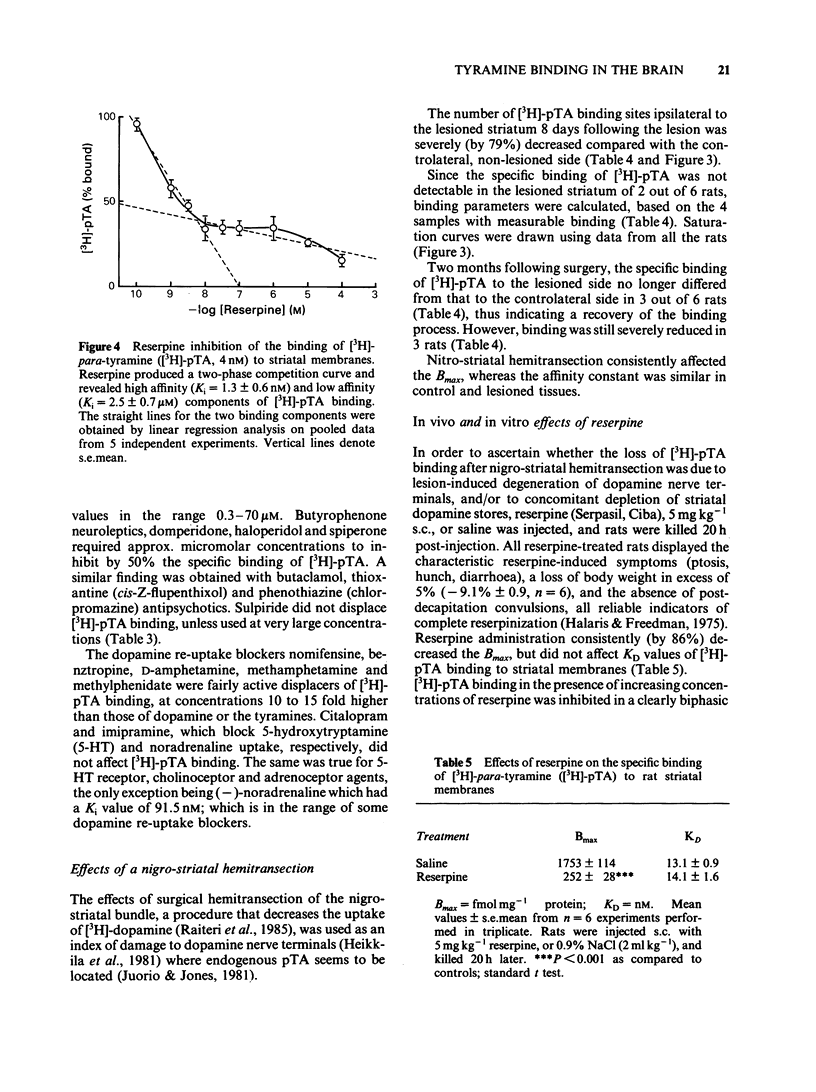
Optimum assay conditions for the association of [3H]-para-tyramine [( 3H]-pTA) with rat brain membranes were characterized, and a saturable, reversible, drug-specific, and high affinity binding mechanism for this trace amine was revealed. The binding capacity (Bmax) for [3H]-pTA in the corpus striatum was approximately 30 times higher than that in the cerebellum, with similar dissociation constants (KD). The binding process of [3H]-pTA involved the dopamine system, inasmuch as (a) highest binding capacity was associated with dopamine-rich regions; (b) dopamine and pTA equally displaced specifically bound [3H]-pTA; (c) there was a severe loss in striatal binding capacity for [3H]-pTA and, reportedly, for [3H]-dopamine, following unilateral nigrostriatal lesion; (d) acute in vivo reserpine treatment markedly decreased the density of [3H]-pTA and, reportedly, of [3H]-dopamine binding sites. In competition experiments [3H]-pTA binding sites, though displaying nanomolar affinity for dopamine, revealed micromolar affinities for the dopamine agonists apomorphine and pergolide, and for several dopamine antagonists, while having very high affinity for reserpine, a marker for the catecholamine transporter in synaptic vesicles. The binding process of [3H]-pTA was both energy-dependent (ouabain-sensitive), and ATP-Mg2+-insensitive; furthermore, the potencies of various drugs in competing for [3H]-pTA binding to rat striatal membranes correlated well (r = 0.96) with their reported potencies in inhibiting [3H]-dopamine uptake into striatal synaptosomes. It is concluded that [3H]-pTA binds at a site located on/within synaptic vesicles where it is involved in the transport mechanism of dopamine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacopoulos N. G. Dopaminergic 3H-agonist receptors in rat brain. New evidence on localization and pharmacology. Life Sci. 1984 Jan 23;34(4):307–315. doi: 10.1016/0024-3205(84)90617-9. [DOI] [PubMed] [Google Scholar]
- Bogdanski D. F., Tissari A., Brodie B. B. Role of sodium, potassium, ouabain and reserpine in uptake, storage and metabolism of biogenic amines in synaptosomes. Life Sci. 1968 Apr 1;7(7):419–428. doi: 10.1016/0024-3205(68)90013-1. [DOI] [PubMed] [Google Scholar]
- Boulton A. A., Juorio A. V., Philips S. R., Wu P. H. The effects of reserpine and 6-hydroxydopamine on the concentrations of some arylakylamines in rat brain. Br J Pharmacol. 1977 Jan;59(1):209–214. doi: 10.1111/j.1476-5381.1977.tb06996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. R., Creese I., Snyder S. H. Properties of [3H]haloperidol and [3H]dopamine binding associated with dopamine receptors in calf brain membranes. Mol Pharmacol. 1976 Sep;12(5):800–812. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Creese I., Usdin T. B., Snyder S. H. Dopamine receptor binding regulated by guanine nucleotides. Mol Pharmacol. 1979 Jul;16(1):69–76. [PubMed] [Google Scholar]
- Halaris A. E., Freedman D. X. Loss of body weight as a predictor of reserpine-induced amine depletion. Eur J Pharmacol. 1975 May;32(1):93–101. doi: 10.1016/0014-2999(75)90327-1. [DOI] [PubMed] [Google Scholar]
- Hall M. D., Jenner P., Kelly E., Marsden C. D. Differential anatomical location of [3H]-N,n-propylnorapomorphine and [3H]-spiperone binding sites in the striatum and substantia nigra of the rat. Br J Pharmacol. 1983 Jun;79(2):599–610. doi: 10.1111/j.1476-5381.1983.tb11035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Manzino L. Stereospecific binding of 3H-dopamine in neostriatal membrane preparations: inhibitory effects of sodium ascorbate. Life Sci. 1983 May 9;32(19):2183–2191. doi: 10.1016/0024-3205(83)90416-2. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Shapiro B. S., Duvoisin R. C. The relationship between loss of dopamine nerve terminals, striatal [3H]spiroperidol binding and rotational behavior in unilaterally 6-hydroxydopamine-lesioned rats. Brain Res. 1981 May 4;211(2):285–292. doi: 10.1016/0006-8993(81)90614-4. [DOI] [PubMed] [Google Scholar]
- Janowsky A., Schweri M. M., Berger P., Long R., Skolnick P., Paul S. M. The effects of surgical and chemical lesions on striatal [3H]threo-(+/-)-methylphenidate binding: correlation with [3H]dopamine uptake. Eur J Pharmacol. 1985 Jan 22;108(2):187–191. doi: 10.1016/0014-2999(85)90724-1. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., Blaustein R. O., Snyder S. H. [3H]mazindol binding associated with neuronal dopamine uptake sites in corpus striatum membranes. Eur J Pharmacol. 1983 Jun 17;90(4):461–462. doi: 10.1016/0014-2999(83)90574-5. [DOI] [PubMed] [Google Scholar]
- Juorio A. V. A possible role for tyramines in brain function and some mental disorders. Gen Pharmacol. 1982;13(3):181–183. doi: 10.1016/0306-3623(82)90087-8. [DOI] [PubMed] [Google Scholar]
- Juorio A. V. Drug-induced changes in the formation, storage and metabolism of tyramine in the mouse. Br J Pharmacol. 1979 Jul;66(3):377–384. doi: 10.1111/j.1476-5381.1979.tb10841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juorio A. V., Jones R. S. The effect of mesencephalic lesions on tyramine and dopamine in the caudate nucleus of the rat. J Neurochem. 1981 Jun;36(6):1898–1903. doi: 10.1111/j.1471-4159.1981.tb10813.x. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Creese I. Dopaminergic D-3 binding sites are not presynaptic autoreceptors. Nature. 1983 Dec 8;306(5943):586–589. doi: 10.1038/306586a0. [DOI] [PubMed] [Google Scholar]
- Lentzen H., Philippu A. Uptake of tyramine into synaptic vesicles of the caudate nucleus. Naunyn Schmiedebergs Arch Pharmacol. 1977 Oct;300(1):25–30. doi: 10.1007/BF00505076. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Petrali E. H., Boulton A. A., Dyck L. E. Uptake of para-tyramine and meta-tyramine into slices of the caudate nucleus and hypothalamus of the rat. Neurochem Res. 1979 Oct;4(5):633–642. doi: 10.1007/BF00964440. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Versace P., Marchi M. GM1 monosialoganglioside inner ester induces early recovery of striatal dopamine uptake in rats with unilateral nigrostriatal lesion. Eur J Pharmacol. 1985 Dec 3;118(3):347–350. doi: 10.1016/0014-2999(85)90146-3. [DOI] [PubMed] [Google Scholar]
- Richelson E., Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol. 1984 Sep 17;104(3-4):277–286. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. Nomenclature of central and peripheral dopaminergic sites and receptors. Biochem Pharmacol. 1982 Aug 15;31(16):2563–2569. doi: 10.1016/0006-2952(82)90700-6. [DOI] [PubMed] [Google Scholar]
- Seeman P., Ulpian C., Grigoriadis D., Pri-Bar I., Buchman O. Conversion of dopamine D1 receptors from high to low affinity for dopamine. Biochem Pharmacol. 1985 Jan 1;34(1):151–154. doi: 10.1016/0006-2952(85)90116-9. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Liem A. L., Mulder A. H. Release and receptor stimulating properties of p-tyramine in rat brain. Arch Int Pharmacodyn Ther. 1976 Mar;220(1):62–71. [PubMed] [Google Scholar]
- Toffano G., Savoini G., Moroni F., Lombardi G., Calza L., Agnati L. F. GM1 ganglioside stimulates the regeneration of dopaminergic neurons in the central nervous system. Brain Res. 1983 Feb 14;261(1):163–166. doi: 10.1016/0006-8993(83)91298-2. [DOI] [PubMed] [Google Scholar]
- Toll L., Gundersen C. B., Jr, Howard B. D. Energy utilization in the uptake of catecholamines by synaptic vesicles and adrenal chromaffin granules. Brain Res. 1977 Nov 4;136(1):59–66. doi: 10.1016/0006-8993(77)90131-7. [DOI] [PubMed] [Google Scholar]
- Ungar F., Mosnaim A. D., Ungar B., Wolf M. E. Tyramine-binding by synaptosomes from rat brain: effect of centrally active drugs. Biol Psychiatry. 1977 Oct;12(5):661–668. [PubMed] [Google Scholar]
- Zivin J. A., Waud D. R. How to analyze binding, enzyme and uptake data: the simplest case, a single phase. Life Sci. 1982 Apr 26;30(17):1407–1422. doi: 10.1016/0024-3205(82)90554-9. [DOI] [PubMed] [Google Scholar]


