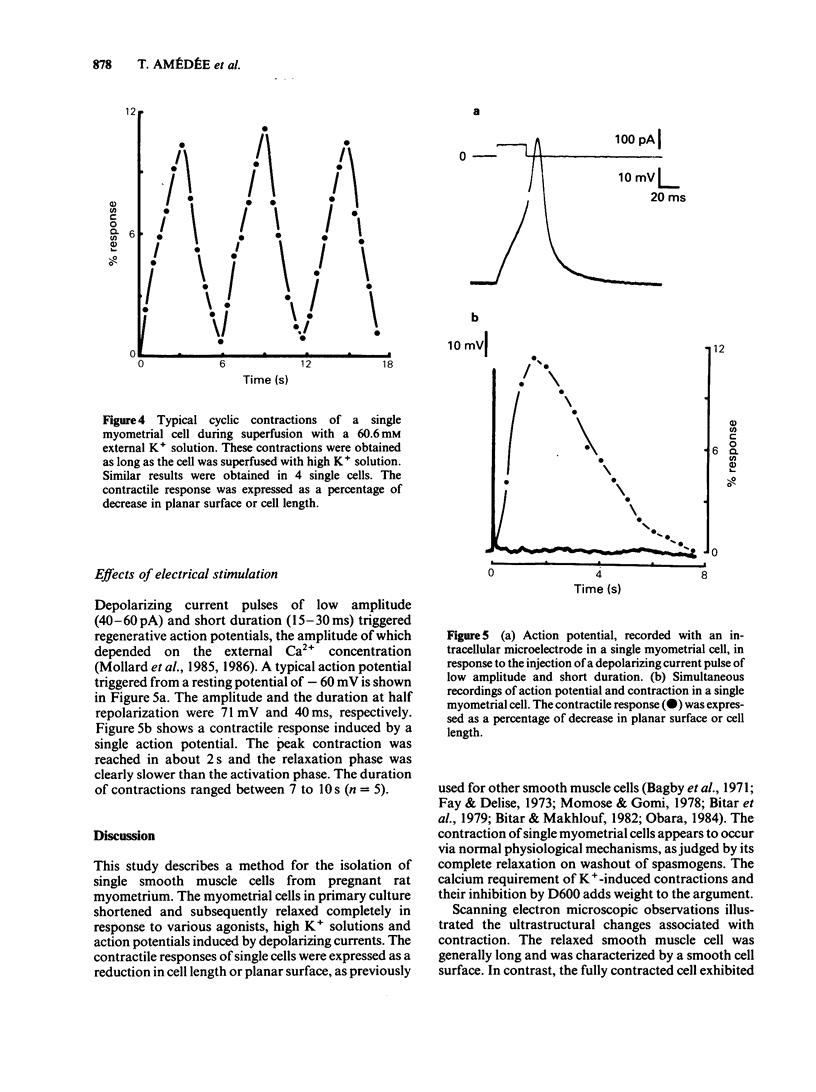
Abstract
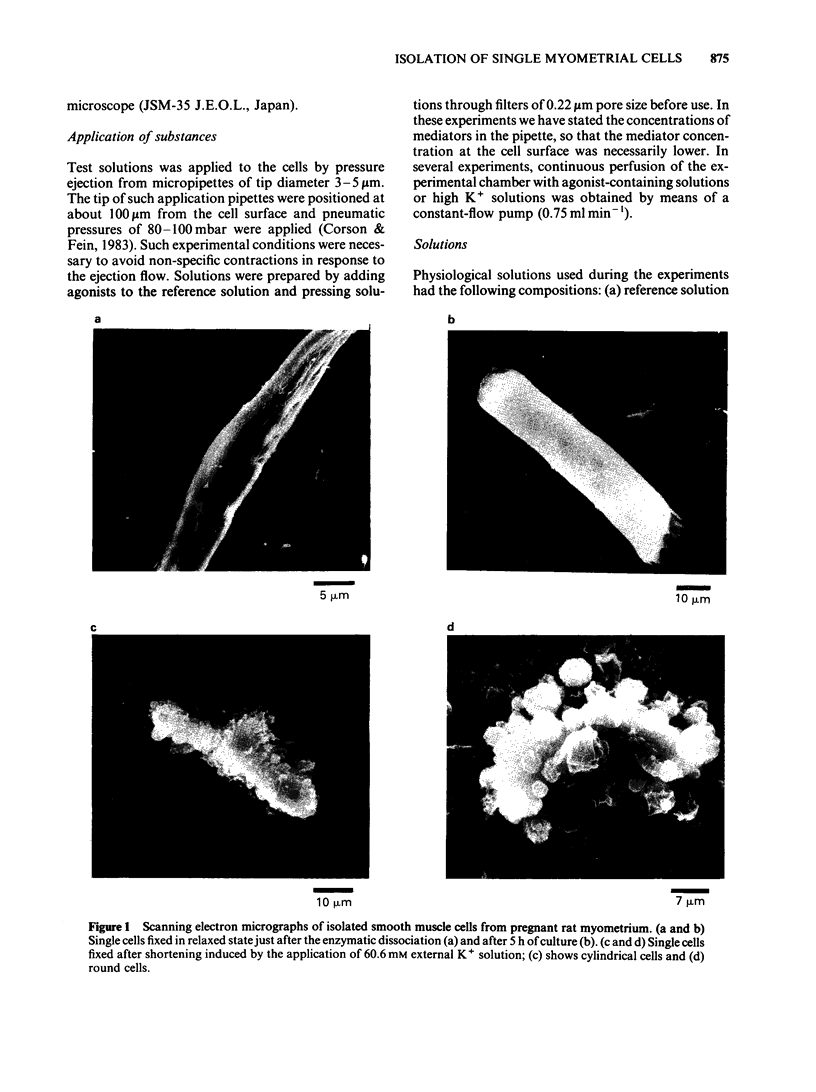
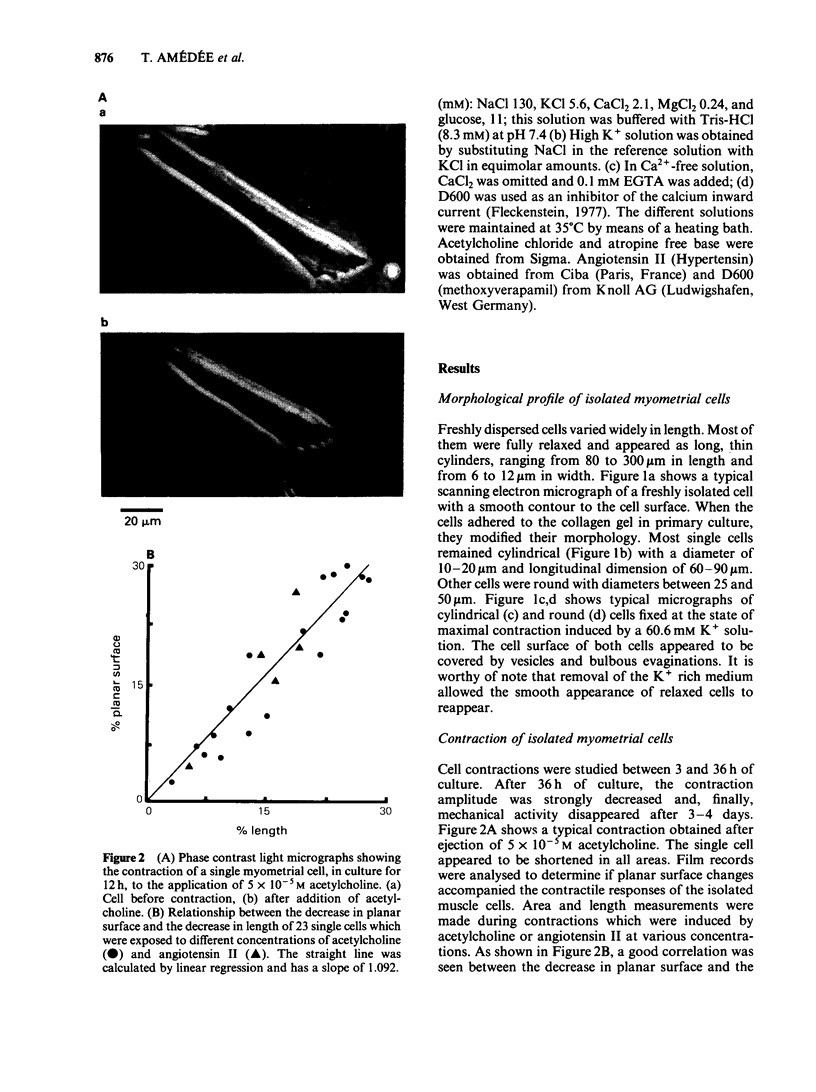
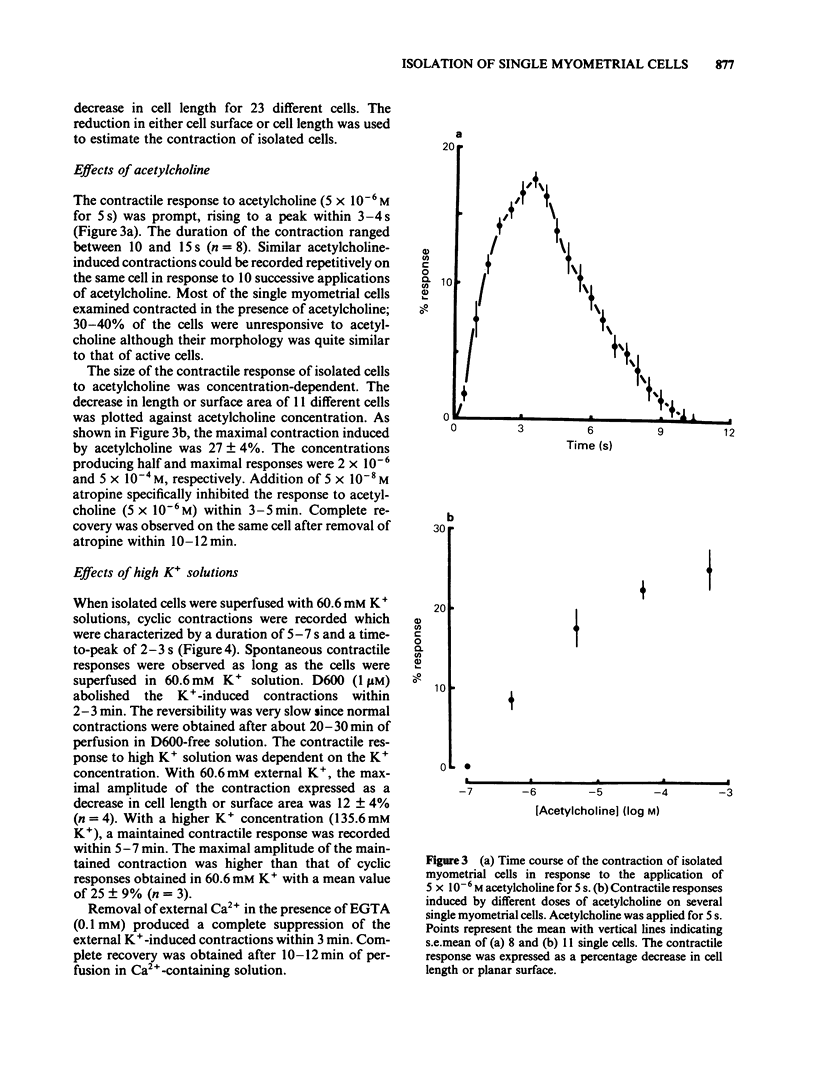
A modified method for enzymatically isolating myometrial cells from the pregnant rat has been developed and the mechanical properties of single cells in short-term primary culture have been studied in response to various stimuli. The dissociation method produced a high proportion of fully relaxed cells and these cells shortened and subsequently relaxed completely in response to successive applications of acetylcholine, angiotensin II, high K+ solution or depolarizing current. In single cells, the contractions induced by acetylcholine and high K+ solution were concentration-dependent. Maximal contractions were obtained with 135.6 mM K+ and 5 X 10(-4)M acetylcholine. In single myometrial cells, the time course of contractions induced by acetylcholine, high K+ solution or depolarizing current was similar, suggesting that the rate of shortening was determined by limits of the contractile mechanism. Scanning electron microscopy revealed a smooth surface to the relaxed cells which contrasted with the numerous evaginations present on fully contracted cells. These results demonstrate the retention of structural integrity, acetylcholine and angiotensin II receptors, and potential-dependent Ca channels in myometrial single cells in short-term primary culture. Cells produced by this technique may provide a useful model for detailed electrophysiological studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Bagby R. M., Young A. M., Dotson R. S., Fisher B. A., McKinnon K. Contraction of single smooth muscle cells from Bufo marinus stomach. Nature. 1971 Dec 10;234(5328):351–352. doi: 10.1038/234351a0. [DOI] [PubMed] [Google Scholar]
- Bengtsson B. The role of intramural noradrenaline in the potassium induced contracture of non-estrogenized smooth muscle. Acta Physiol Scand. 1977 Sep;101(1):112–121. doi: 10.1111/j.1748-1716.1977.tb05989.x. [DOI] [PubMed] [Google Scholar]
- Bitar K. N., Makhlouf G. M. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. Am J Physiol. 1982 Apr;242(4):G400–G407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- Bitar K. N., Zfass A. M., Makhlouf G. M. Interaction of acetylcholine and cholecystokinin with dispersed smooth muscle cells. Am J Physiol. 1979 Aug;237(2):E172–E176. doi: 10.1152/ajpendo.1979.237.2.E172. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Corson D. W., Fein A. Quantitative pressure injection of picoliter volumes into Limulus ventral photoreceptors. Biophys J. 1983 Dec;44(3):299–304. doi: 10.1016/S0006-3495(83)84303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Responses of enzymatically isolated mammalian vascular smooth muscle cells to pharmacological and electrical stimuli. Pflugers Arch. 1985 May;404(1):100–102. doi: 10.1007/BF00581502. [DOI] [PubMed] [Google Scholar]
- Fay F. S., Delise C. M. Contraction of isolated smooth-muscle cells--structural changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):641–645. doi: 10.1073/pnas.70.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Singer J. J. Characteristics of response of isolated smooth muscle cells to cholinergic drugs. Am J Physiol. 1977 Mar;232(3):C144–C154. doi: 10.1152/ajpcell.1977.232.3.C144. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Gabella G. Effect of potassium on the mechanical activity of taenia coli, uterus and portal vein of the guinea-pig. Q J Exp Physiol Cogn Med Sci. 1978 Apr;63(2):125–146. doi: 10.1113/expphysiol.1978.sp002426. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Schultz G. S., Galardy R. E., Jamieson J. D. Preparation of functional smooth muscle cells from the rabbit aorta. J Exp Med. 1978 Nov 1;148(5):1400–1413. doi: 10.1084/jem.148.5.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Ogasawara T., Osa T. Calcium diffusion in uterine smooth muscle sheets. J Gen Physiol. 1982 Aug;80(2):257–277. doi: 10.1085/jgp.80.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL J. M. Regulation of activity in uterine smooth muscle. Physiol Rev Suppl. 1962 Jul;5:213–227. [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Mironneau C., Grosset A., Hamon G., Savineau J. P. Action of angiotensin II on the electrical and mechanical activity of rat uterine smooth muscle. Eur J Pharmacol. 1980 Dec 5;68(3):275–285. doi: 10.1016/0014-2999(80)90525-7. [DOI] [PubMed] [Google Scholar]
- Mollard P., Mironneau J., Amedee T., Mironneau C. Electrophysiological characterization of single pregnant rat myometrial cells in short-term primary culture. Am J Physiol. 1986 Jan;250(1 Pt 1):C47–C54. doi: 10.1152/ajpcell.1986.250.1.C47. [DOI] [PubMed] [Google Scholar]
- Obara K. Isolation and contractile properties of single smooth muscle cells from guinea pig taenia caeci. Jpn J Physiol. 1984;34(1):41–54. doi: 10.2170/jjphysiol.34.41. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Passive properties of the membrane of single freshly isolated smooth muscle cells. Am J Physiol. 1980 Nov;239(5):C153–C161. doi: 10.1152/ajpcell.1980.239.5.C153. [DOI] [PubMed] [Google Scholar]
- Van Dijk A. M., Laird J. D. Characterization of single isolated vascular smooth muscle cells from bovine coronary artery. Blood Vessels. 1984;21(6):267–278. doi: 10.1159/000158529. [DOI] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Voltage clamp of single freshly dissociated smooth muscle cells: current-voltage relationships for three currents. Pflugers Arch. 1981 May;390(2):207–210. doi: 10.1007/BF00590209. [DOI] [PubMed] [Google Scholar]