Abstract
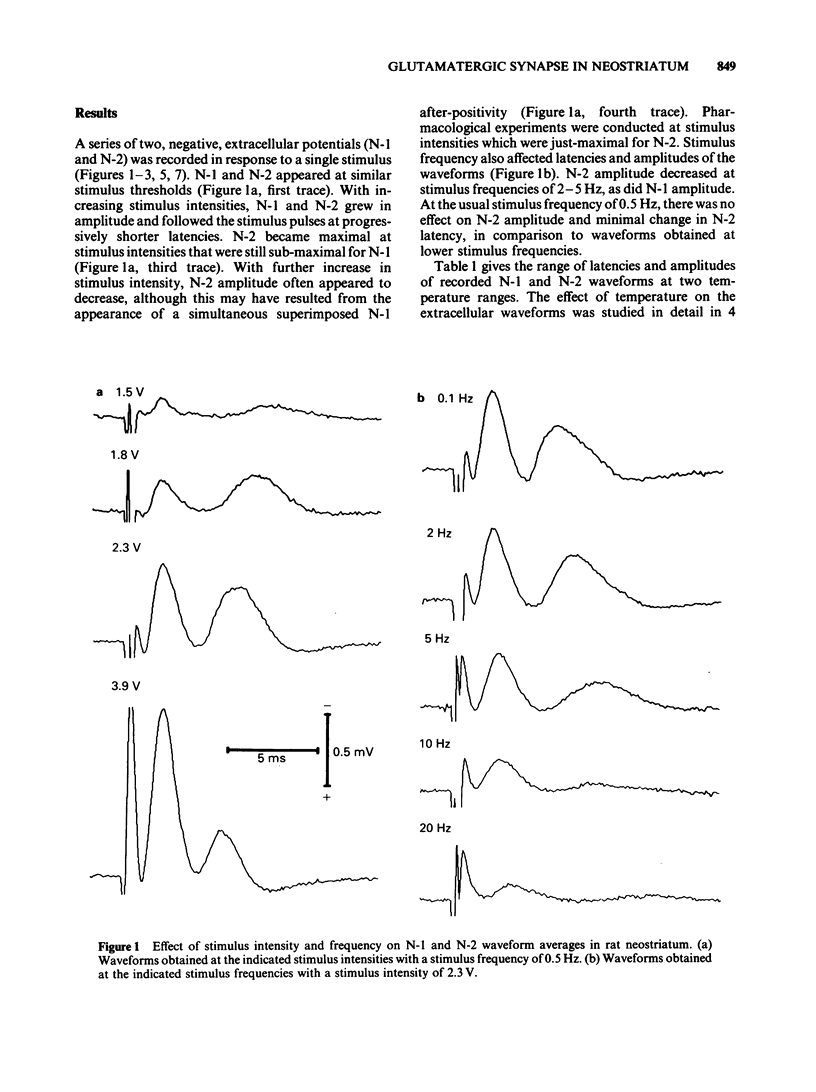
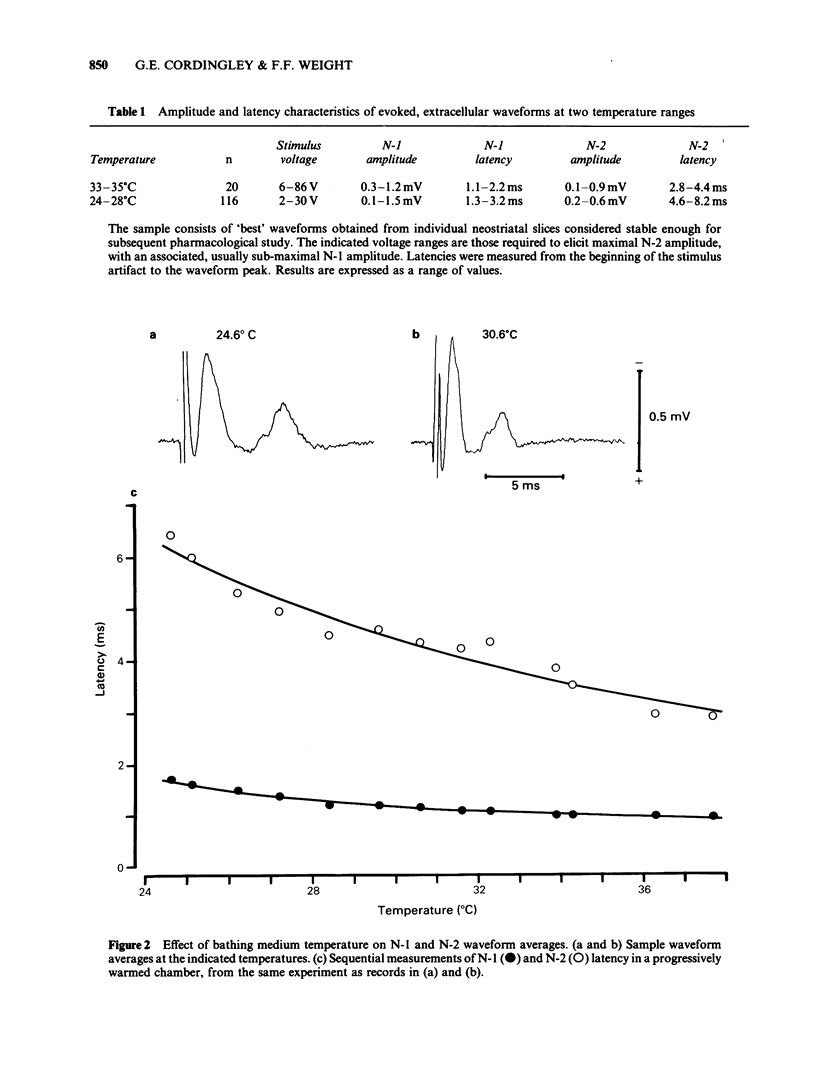
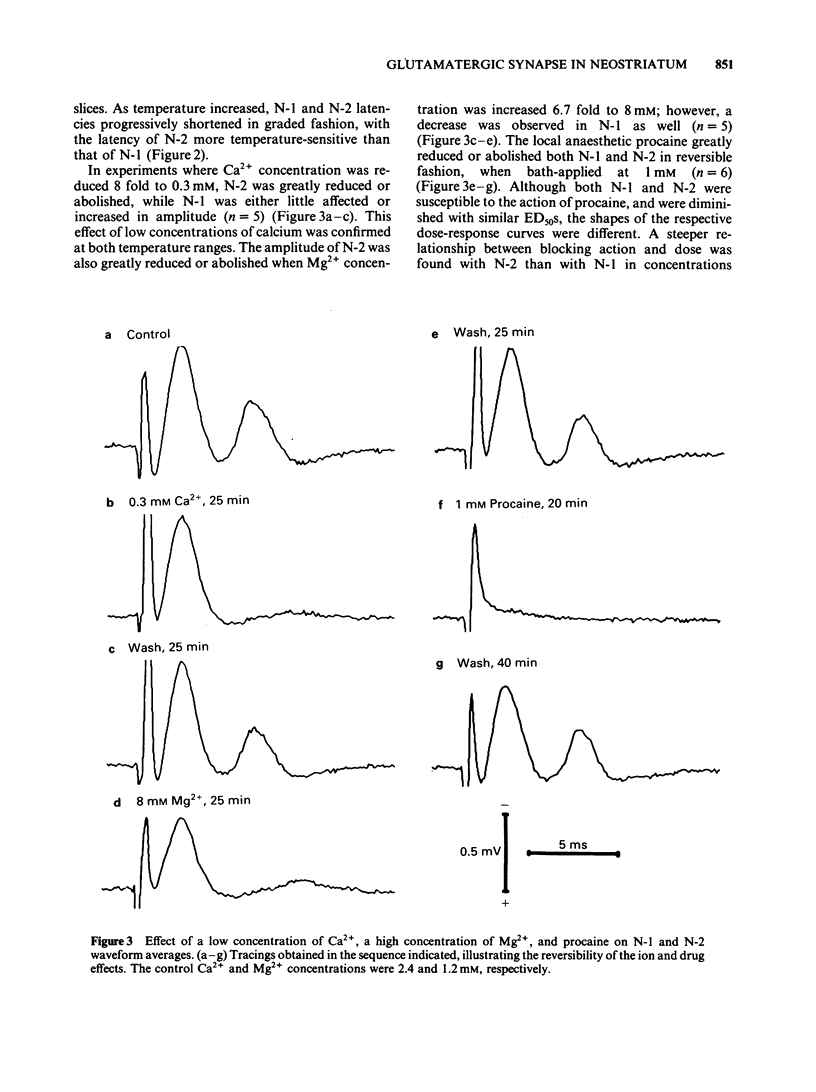
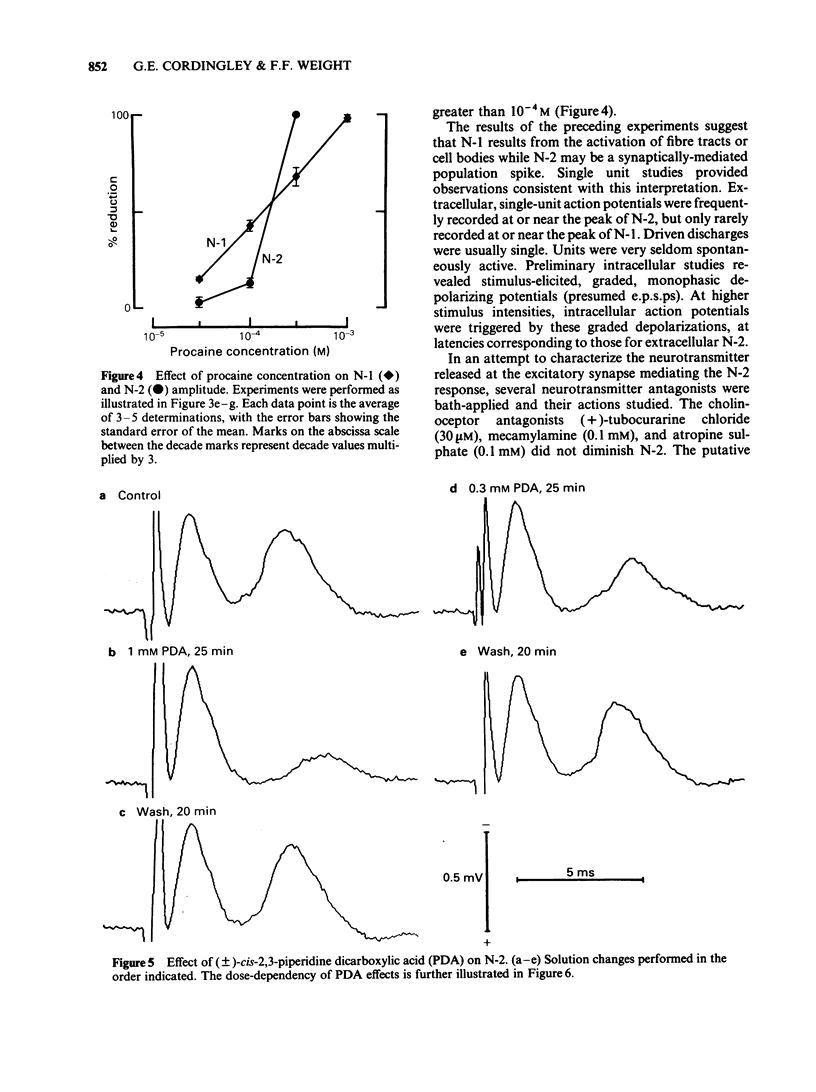
We studied the synaptic pharmacology of an excitatory pathway in the neostriatum using electrophysiological techniques in tissue slices from rats. In response to single electrical stimuli, two negative, extracellular potentials (N-1 and N-2) were recorded through micropipette electrodes within 150-450 micron of the stimulating cathode. N-2 was reversibly reduced or abolished by reducing the concentration of calcium in the bathing medium, while N-1 was unaffected. Both N-1 and N-2 were reversibly abolished by the local anaesthetic procaine. Single-unit, extracellular action potentials were, at times, associated with either N-1 or N-2. Intracellular recordings showed action potentials at N-2 latency arising from graded, monophasic, depolarizing potentials. Bath-applied cholinoceptor and dopamine receptor antagonists failed to reduce N-2. By contrast, antagonists of excitatory amino acid transmitters reversibly reduced or abolished N-2. gamma-D-Glutamylglycine (GG), (+/-)-cis-2,3-piperidine dicarboxylic acid (PDA) and DL-2-amino-4-phosphonobutyric acid (APB) blocked N-2 with ED50S of 0.79 mM, 1.0 mM and 1.1 mM, respectively. (-)-Baclofen reversibly blocked N-2 with an ED50 of 0.79 microM; (+)-baclofen was 330 times less potent. The results suggest that N-1 results from direct activation of fibre tracts or cell bodies, while N-2 is a population spike mediated by excitatory synapses whose natural transmitter pharmacologically resembles glutamate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B., Evans R. H. The depressant action of baclofen on the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1981 May 22;71(4):357–364. doi: 10.1016/0014-2999(81)90179-5. [DOI] [PubMed] [Google Scholar]
- Ault B., Nadler J. V. Baclofen selectively inhibits transmission at synapses made by axons of CA3 pyramidal cells in the hippocampal slice. J Pharmacol Exp Ther. 1982 Nov;223(2):291–297. [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- CARMAN J. B., COWAN W. M., POWELL T. P. THE ORGANIZATION OF CORTICO-STRIATE CONNEXIONSIN THE RABBIT. Brain. 1963 Sep;86:525–562. doi: 10.1093/brain/86.3.525. [DOI] [PubMed] [Google Scholar]
- Cain C. R., Simmonds M. A. Effects of baclofen on the olfactory cortex slice preparation. Neuropharmacology. 1982 Apr;21(4):371–373. doi: 10.1016/0028-3908(82)90103-4. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Anson J., Kelly E. P. Baclofen: effects on evoked field potentials and amino acid neurotransmitter release in the rat olfactory cortex slice. Brain Res. 1982 Apr 29;238(2):371–383. doi: 10.1016/0006-8993(82)90111-1. [DOI] [PubMed] [Google Scholar]
- Collins G. G. Some effects of excitatory amino acid receptor antagonists on synaptic transmission in the rat olfactory cortex slice. Brain Res. 1982 Jul 29;244(2):311–318. doi: 10.1016/0006-8993(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Sears E. S. The effects of Lioresal on synaptic activity in the isolated spinal cord. Neurology. 1974 Oct;24(10):957–963. doi: 10.1212/wnl.24.10.957. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Davies J. Selective depression of synaptic transmission of spinal neurones in the cat by a new centrally acting muscle relaxant, 5-chloro-4-(2-imidazolin-2-yl-amino)-2, 1, 3-benzothiodazole (DS103-282). Br J Pharmacol. 1982 Jul;76(3):473–481. doi: 10.1111/j.1476-5381.1982.tb09242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I., Fonnum F., Storm-Mathisen J. High affinity uptake of glutamate in terminals of corticostriatal axons. Nature. 1977 Mar 24;266(5600):377–378. doi: 10.1038/266377a0. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Jones A. W., Smith D. A., Watkins J. C. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982 Jan;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Fromm G. H., Terrence C. F., Chattha A. S., Glass J. D. Baclofen in trigeminal neuralgia: its effect on the spinal trigeminal nucleus: a pilot study. Arch Neurol. 1980 Dec;37(12):768–771. doi: 10.1001/archneur.1980.00500610048007. [DOI] [PubMed] [Google Scholar]
- Galindo A. GABA-picrotoxin interaction in the mammalian central nervous system. Brain Res. 1969 Aug;14(3):763–767. doi: 10.1016/0006-8993(69)90220-0. [DOI] [PubMed] [Google Scholar]
- Glees P. The anatomical basis of cortico-striate connexions. J Anat. 1944 Jan;78(Pt 1-2):47–51. [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Kato M., Waldmann U., Murakami S. Effects of baclofen on spinal neurones of cats. Neuropharmacology. 1978 Oct;17(10):827–833. doi: 10.1016/0028-3908(78)90071-0. [DOI] [PubMed] [Google Scholar]
- Kemp J. M., Powell T. P. The cortico-striate projection in the monkey. Brain. 1970;93(3):525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kemp J. M., Powell T. P. The site of termination of afferent fibres in the caudate nucleus. Philos Trans R Soc Lond B Biol Sci. 1971 Sep 30;262(845):413–427. doi: 10.1098/rstb.1971.0104. [DOI] [PubMed] [Google Scholar]
- Kim J. S., Hasller R., Hau P., Paik K. S. Effect of frontal cortex ablation on striatal glutamic acid level in rat. Brain Res. 1977 Aug 26;132(2):370–374. doi: 10.1016/0006-8993(77)90430-9. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Scherer U., Singh K. A glutamatergic corticostriatal path? Brain Res. 1977 Jun 10;128(2):369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Okada Y., Hassler R. Locally evoked potentials in slices of rat neostriatum: a tool for the investigation of intrinsic excitatory processes. Exp Brain Res. 1979 Feb 15;34(3):575–590. doi: 10.1007/BF00239150. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Weiler M. H., Bak I. J. Intrinsic cholinergic excitation in the rat neostriatum: nicotinic and muscarinic receptors. Exp Brain Res. 1980;39(4):401–409. doi: 10.1007/BF00239304. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Baudry M., Fagni L., Lynch G. The blocking action of baclofen on excitatory transmission in the rat hippocampal slice. J Neurosci. 1982 Jun;2(6):698–703. doi: 10.1523/JNEUROSCI.02-06-00698.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- Potashner S. J., Gerard D. Kainate-enhanced release of D-[3H]aspartate from cerebral cortex and striatum: reversal by baclofen and pentobarbital. J Neurochem. 1983 Jun;40(6):1548–1557. doi: 10.1111/j.1471-4159.1983.tb08125.x. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. The excitation and inhibition of crustacean muscle by amino acids. J Physiol. 1959 Oct;148:39–50. doi: 10.1113/jphysiol.1959.sp006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C., Cuenod M. Glutamate release in vitro from corticostriatal terminals. Brain Res. 1979 Oct 26;176(1):185–188. doi: 10.1016/0006-8993(79)90884-9. [DOI] [PubMed] [Google Scholar]
- WEBSTER K. E. Cortico-striate interrelations in the albino rat. J Anat. 1961 Oct;95:532–544. [PMC free article] [PubMed] [Google Scholar]
- Zbicz K. L., Weight F. F. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985 Apr;53(4):1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]


