Abstract
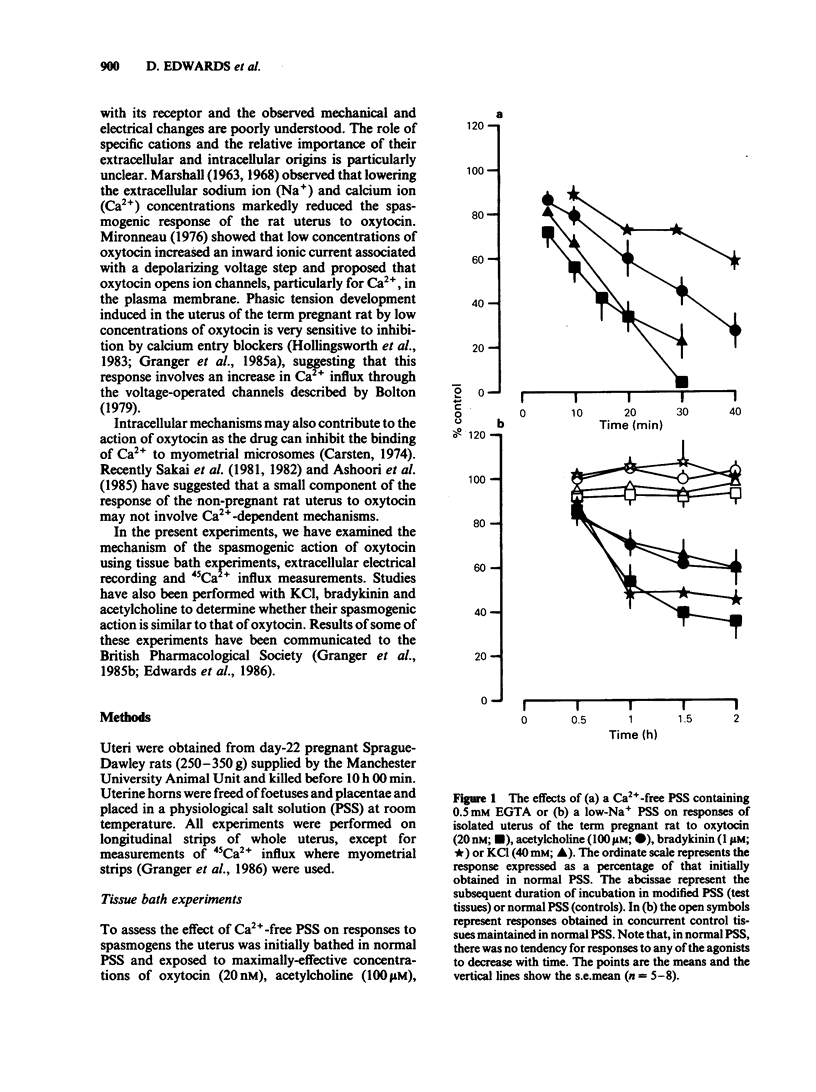
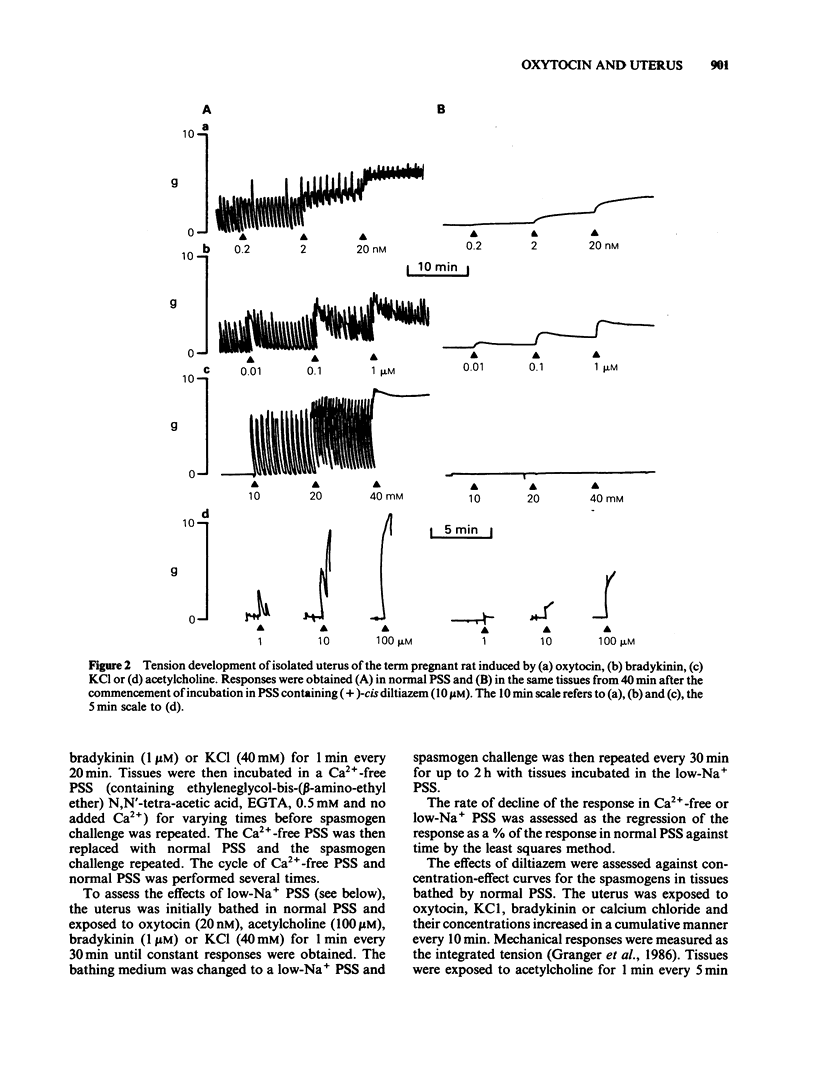
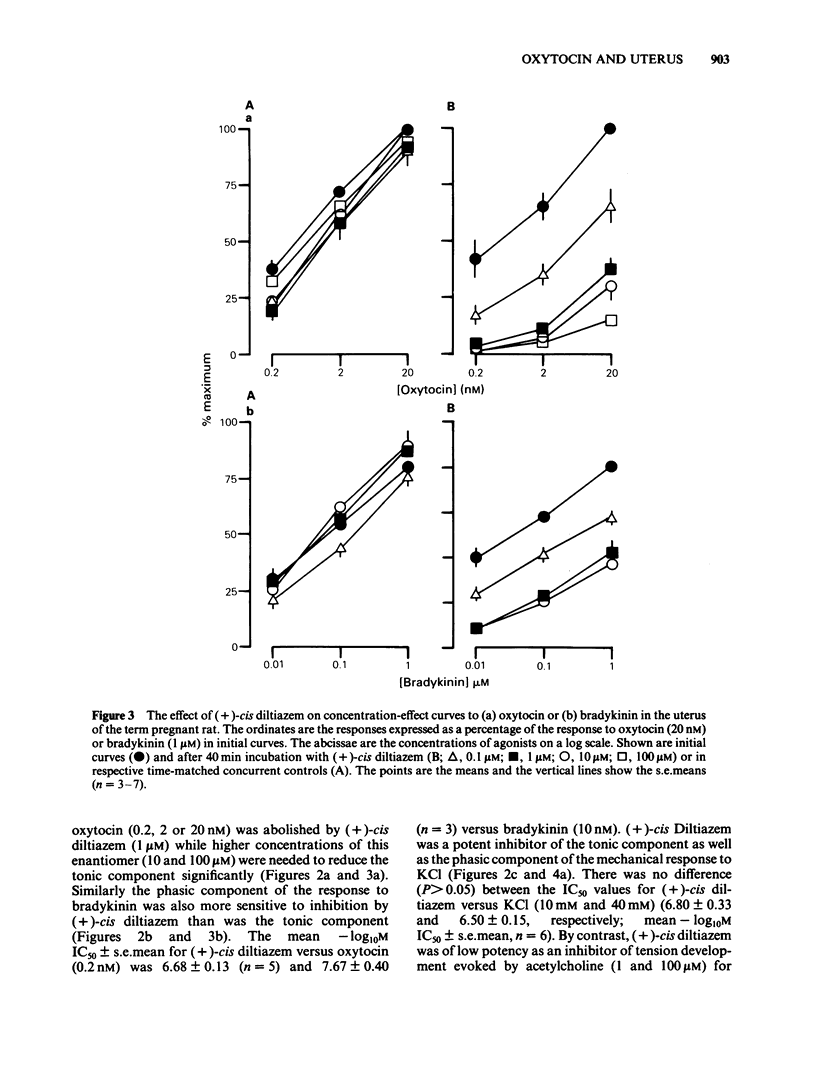
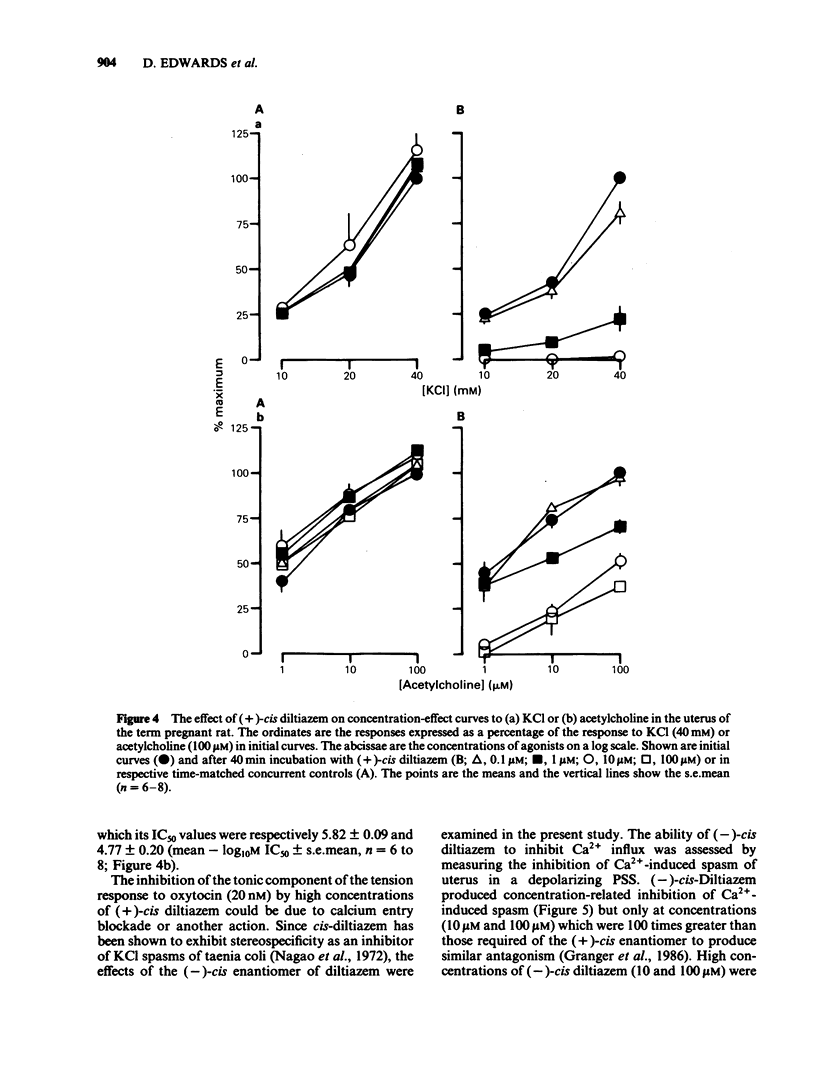
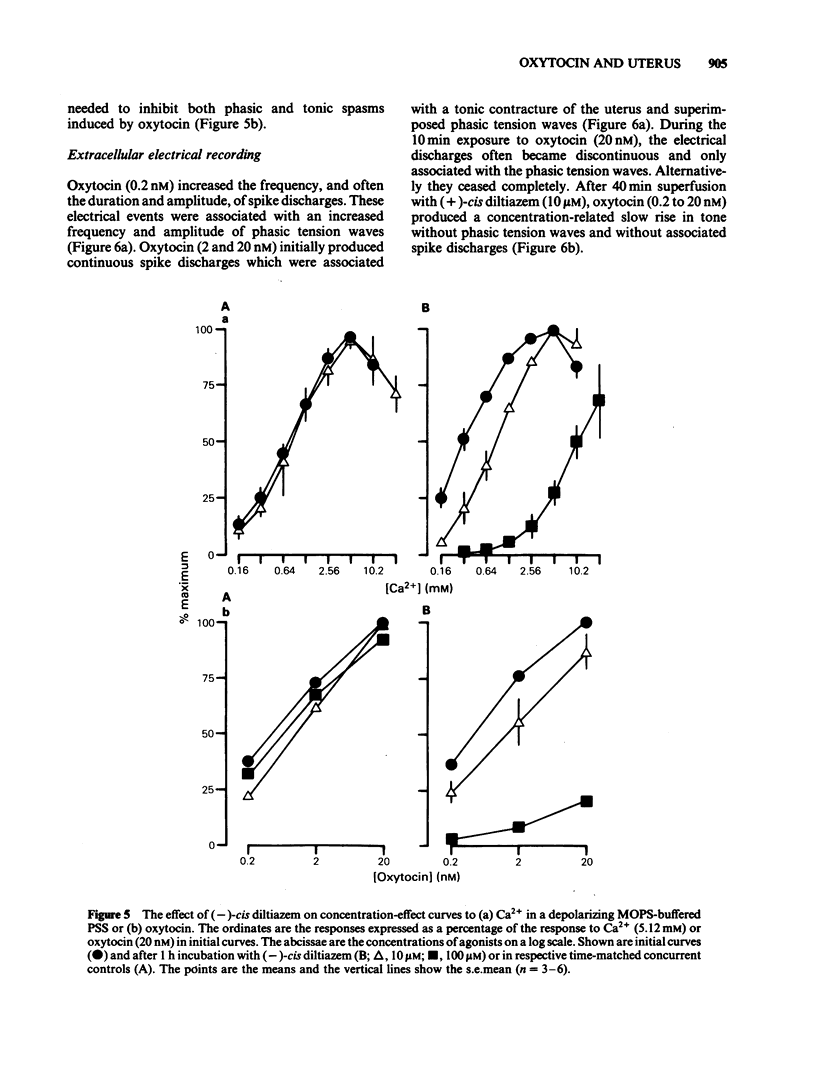
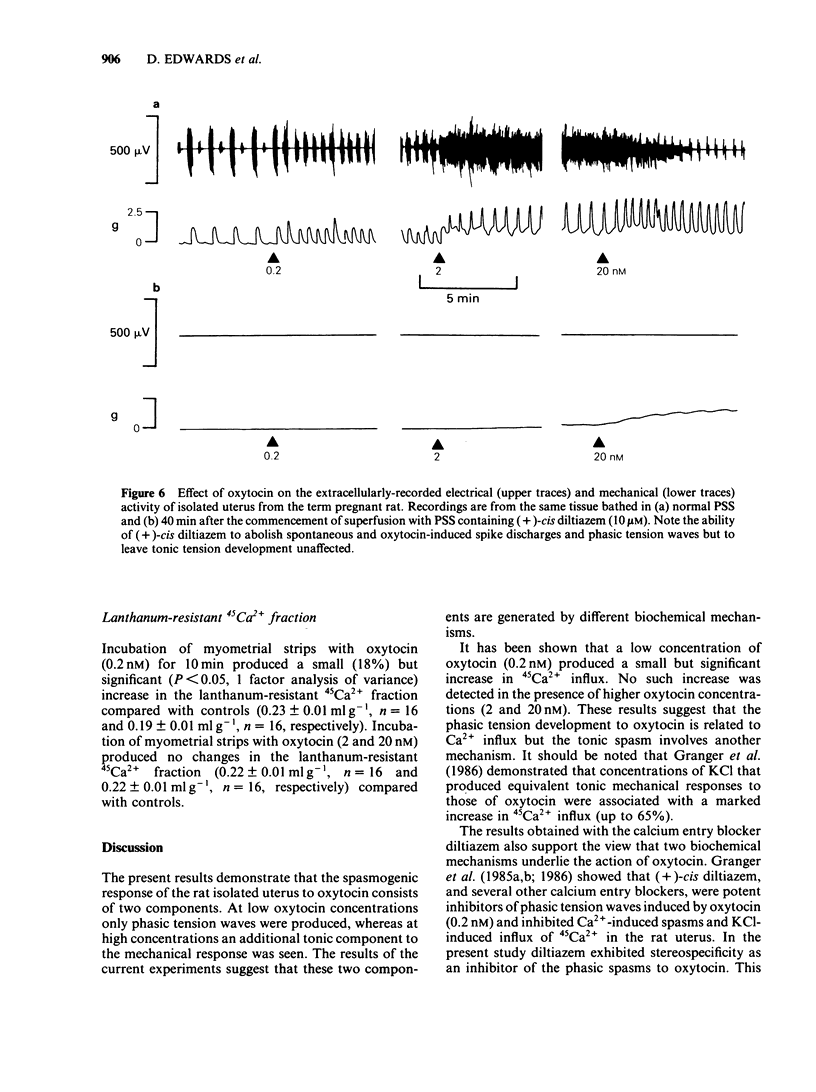
A low concentration (0.2 nM) of oxytocin induced phasic tension development in the isolated uterus of the day-22 pregnant rat. Tonic spasm was also induced by higher concentrations of oxytocin (2 and 20 nM). Spasmogenic responses to bradykinin and potassium chloride (KCl) also contained phasic and tonic components while acetylcholine induced tonic spasm only. The phasic component of the responses to oxytocin and to bradykinin and both components of the response to KCl were inhibited by (+)-cis diltiazem (0.1 and 1 microM). The tonic component of the responses to oxytocin and to bradykinin and the responses to acetylcholine were only reduced by (+)-cis diltiazem at concentrations greater than 10 microM. (-)-cis Diltiazem was less potent than (+)-cis diltiazem as an inhibitor of calcium (Ca2+)-induced spasm in a depolarizing medium and of the phasic spasms induced by oxytocin. The two isomers were of similar potency as inhibitors of oxytocin-induced tonic spasm. Spasmogenic responses to oxytocin, bradykinin, acetylcholine and KCl were decreased when uteri were bathed in media which were Ca2+-free or of low Na+ content. However, there was no correlation between the rank order of sensitivity of the four spasmogens to the changed media and to their inhibition by (+)-cis diltiazem. Oxytocin (0.2 nM) increased the frequency, duration and amplitude of spike activity, measured by extracellular electrical recording, in parallel with enhancement of phasic tension development. With higher concentrations of oxytocin (2 and 20 nM) spike firing was initially continuous but often subsequently ceased despite the associated tonic contracture. After incubation in (+)-cis diltiazem (10 microM), oxytocin (0.2, 2 and 20 nM) produced graded tonic spasm without spike activity. Oxytocin (0.2 nM) produced a small increase in 45Ca2+ influx into myometrium as assessed by the 'lanthanum method'. Higher concentrations of oxytocin (2 and 20 nM) did not increase 45Ca2+ influx. It is concluded that the phasic component of the response of the uterus to oxytocin and bradykinin is associated with Ca2+ influx via voltage-dependent Ca2+ channels. The tonic component is due to another mechanism(s) which does not appear to involve Ca2+ influx. All of the spasmogenic response to KCl can be explained by Ca2+ influx through voltage-dependent Ca2+ channels. These channels do not appear to be involved in the spasmogenic response to acetylcholine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed F., Foster R. W., Small R. C. Some effects of nifedipine in guinea-pig isolated trachealis. Br J Pharmacol. 1985 Apr;84(4):861–869. doi: 10.1111/j.1476-5381.1985.tb17380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F., Foster R. W., Small R. C., Weston A. H. Some features of the spasmogenic actions of acetylcholine and histamine in guinea-pig isolated trachealis. Br J Pharmacol. 1984 Sep;83(1):227–233. doi: 10.1111/j.1476-5381.1984.tb10139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoori F., Takai A., Tomita T. The response of non-pregnant rat myometrium to oxytocin in Ca-free solution. Br J Pharmacol. 1985 Jan;84(1):175–183. [PMC free article] [PubMed] [Google Scholar]
- Bengtsson B., Chow E. M., Marshall J. M. Calcium dependency of pregnant rat myometrium: comparison of circular and longitudinal muscle. Biol Reprod. 1984 May;30(4):869–878. doi: 10.1095/biolreprod30.4.869. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Carsten M. E. Prostaglandins and oxytocin: their effects on uterine smooth muscle. Prostaglandins. 1974 Jan 10;5(1):33–40. doi: 10.1016/s0090-6980(74)80128-0. [DOI] [PubMed] [Google Scholar]
- Golenhoffen K., von Loh D. Elektrophysiologische Untersuchungen zur normalen Spontanaktivität der isolierten Taenia coli des Meerschweinchens. Pflugers Arch. 1970;314(4):312–328. doi: 10.1007/BF00592289. [DOI] [PubMed] [Google Scholar]
- Granger S. E., Hollingsworth M., Weston A. H. A comparison of several calcium antagonists on uterine, vascular and cardiac muscles from the rat. Br J Pharmacol. 1985 May;85(1):255–262. doi: 10.1111/j.1476-5381.1985.tb08854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger S. E., Hollingsworth M., Weston A. H. Effects of calcium entry blockers on tension development and calcium influx in rat uterus. Br J Pharmacol. 1986 Jan;87(1):147–156. doi: 10.1111/j.1476-5381.1986.tb10166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. Relation between the ionic environment and the action of drugs on the myometrium. Fed Proc. 1968 Jan-Feb;27(1):115–119. [PubMed] [Google Scholar]
- Mironneau J. Effects of oxytocin on ionic currents underlying rhythmic activity and contraction in uterine smooth muscle. Pflugers Arch. 1976 May 12;363(2):113–118. doi: 10.1007/BF01062278. [DOI] [PubMed] [Google Scholar]
- Nagao T., Sato M., Iwasawa Y., Takada T., Ishida R. Studies on a new 1,5-benzothiazepine derivative (CRD-401). 3. Effects of optical isomers of CRD-401 on smooth muscle and other pharmacological properties. Jpn J Pharmacol. 1972 Aug;22(4):467–478. doi: 10.1254/jjp.22.467. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Horowitz J. D. Calcium antagonists: a new class of drugs. Pharmacol Ther. 1983;20(2):203–262. doi: 10.1016/0163-7258(83)90040-2. [DOI] [PubMed] [Google Scholar]
- Reiner O., Marshall J. M. Action of prostaglandin, PGF2alpha, on the uterus of the pregnant rat. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(3):243–250. doi: 10.1007/BF00517384. [DOI] [PubMed] [Google Scholar]
- Sakai K., Higuchi K., Yamaguchi T., Uchida M. Oxytocin-induced Ca-free contraction of rat uterine smooth muscle: effects of preincubation with EGTA and drugs. Gen Pharmacol. 1982;13(5):393–400. doi: 10.1016/0306-3623(82)90104-5. [DOI] [PubMed] [Google Scholar]
- Sakai K., Yamaguchi T., Uchida M. Oxytocin-induced Ca-free contraction of rat uterine smooth muscle: effects of divalent cations and drugs. Arch Int Pharmacodyn Ther. 1981 Mar;250(1):40–54. [PubMed] [Google Scholar]
- Schoemaker H., Langer S. Z. [3H]diltiazem binding to calcium channel antagonists recognition sites in rat cerebral cortex. Eur J Pharmacol. 1985 May 8;111(2):273–277. doi: 10.1016/0014-2999(85)90768-x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kuriyama H. Comparison between prostaglandin E2 and oxytocin actions on pregnant mouse myometrium. Jpn J Physiol. 1975;25(3):345–356. doi: 10.2170/jjphysiol.25.345. [DOI] [PubMed] [Google Scholar]


