Abstract
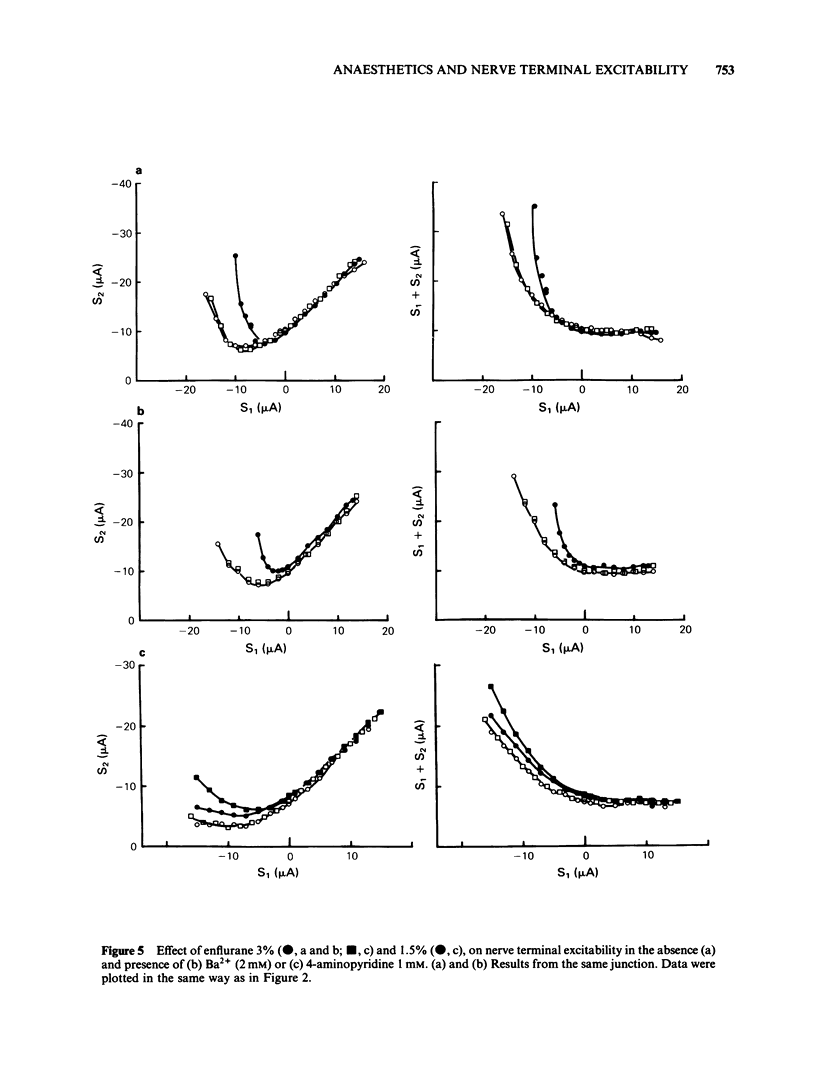
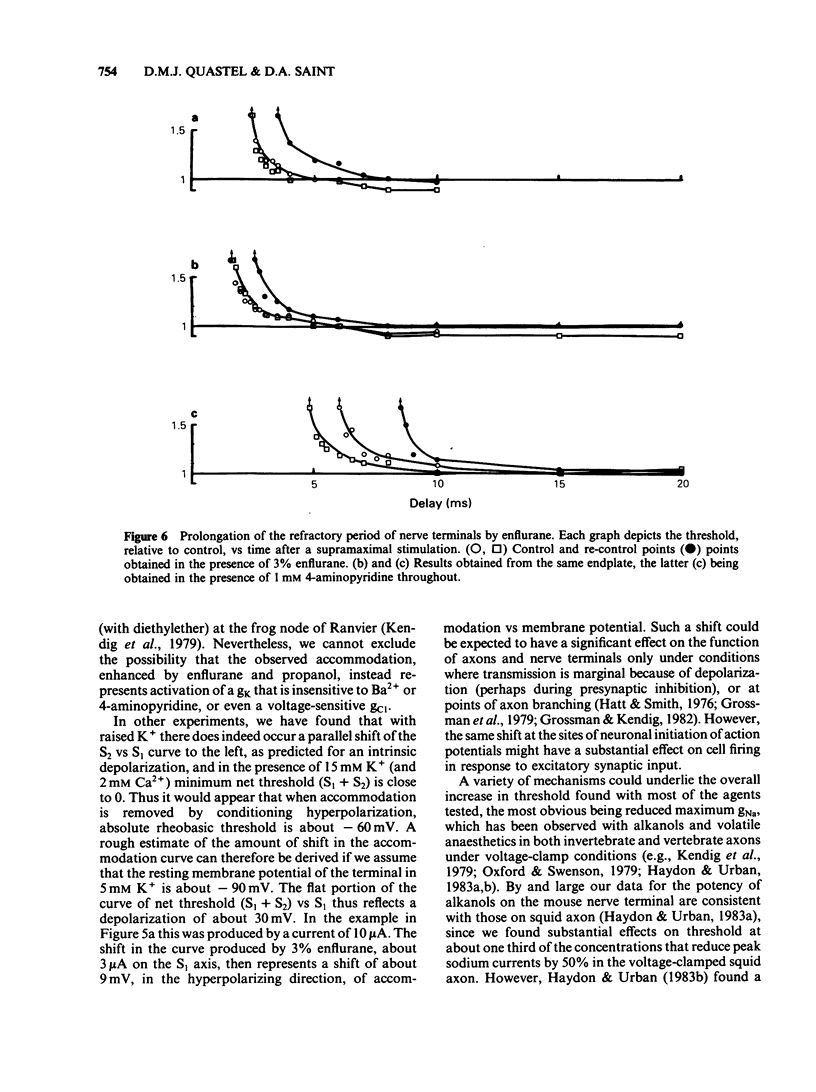
A method of local polarization-excitation was used to study changes in motor nerve terminal excitability produced by n-alkanols and volatile anaesthetics in mouse diaphragm preparations. Ethanol and propanol caused an exaggeration of 'accommodation', i.e., the increase in excitation threshold produced by a conditioning depolarization. Butanol, hexanol and octanol had mixed effects, producing a rise in the minimum threshold (threshold after removal of resting accommodation) in addition to an increase in accommodation. Volatile anaesthetics produced effects on excitability at concentrations comparable to minimum alveolar concentration. The action of enflurane was essentially only to increase accommodation while methoxyflurane produced an increase in threshold insensitive to conditioning polarization. Halothane and isoflurane produced intermediate effects. Accommodation curves were little affected by Ba2+ or 4-aminopyridine and were consistent with accommodation being a reflection of inactivation of the Na+ current system. We conclude that volatile anaesthetics, at concentrations comparable to those producing anaesthesia, may substantially modify Na+ channel gating and inactivation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allott P. R., Steward A., Flook V., Mapleson W. W. Variation with temperature of the solubilities of inhaled anaesthetics in water, oil and biological media. Br J Anaesth. 1973 Mar;45(3):294–300. doi: 10.1093/bja/45.3.294. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Shrager P., Goldstein D. A. Modification of sodium and potassium channel gating kinetics by ether and halothane. J Gen Physiol. 1981 Mar;77(3):233–253. doi: 10.1085/jgp.77.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Polc P. Low-dose benzodiazepine neuronal inhibition: enhanced Ca2+-mediated K+-conductance. Brain Res. 1983 Jul 25;271(2):358–364. doi: 10.1016/0006-8993(83)90302-5. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M., Rogart R. B., Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol. 1979 Jul;292:149–166. doi: 10.1113/jphysiol.1979.sp012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gion H., Saidman L. J. The minimum alveolar concentration of enflurane in man. Anesthesiology. 1971 Oct;35(4):361–364. doi: 10.1097/00000542-197110000-00008. [DOI] [PubMed] [Google Scholar]
- Grossman Y., Kendig J. J. General anesthetic block of a bifurcating axon. Brain Res. 1982 Aug 5;245(1):148–153. doi: 10.1016/0006-8993(82)90350-x. [DOI] [PubMed] [Google Scholar]
- Grossman Y., Parnas I., Spira M. E. Mechanisms involved in differential conduction of potentials at high frequency in a branching axon. J Physiol. 1979 Oct;295:307–322. doi: 10.1113/jphysiol.1979.sp012970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E. J., Macdonald R. L. Barbiturate reduction of calcium-dependent action potentials: correlation with anesthetic action. Brain Res. 1982 Mar 18;236(1):157–171. doi: 10.1016/0006-8993(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Kendig J. J., Courtney K. R., Cohen E. N. Anesthetics: molecular correlates of voltage- and frequency-dependent sodium channel block in nerve. J Pharmacol Exp Ther. 1979 Sep;210(3):446–452. [PubMed] [Google Scholar]
- Oxford G. S., Swenson R. P. n-Alkanols potentiate sodium channel inactivation in squid giant axons. Biophys J. 1979 Jun;26(3):585–590. doi: 10.1016/S0006-3495(79)85273-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelhate M., Pichon Y. Proceedings: Selective inhibition of potassium current in the giant axon of the cockroach. J Physiol. 1974 Oct;242(2):90P–91P. [PubMed] [Google Scholar]
- Richards C. D., White A. E. The actions of volatile anaesthetics on synaptic transmission in the dentate gyrus. J Physiol. 1975 Oct;252(1):241–257. doi: 10.1113/jphysiol.1975.sp011142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidman L. J., Eger E. I., 2nd, Munson E. S., Babad A. A., Muallem M. Minimum alveolar concentrations of methoxyflurane, halothane, ether and cyclopropane in man: correlation with theories of anesthesia. Anesthesiology. 1967 Nov-Dec;28(6):994–1002. doi: 10.1097/00000542-196711000-00009. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Stevens W. C., Dolan W. M., Gibbons R. T., White A., Eger E. I., Miller R. D., DeJong R. H., Elashoff R. M. Minimum alveolar concentrations (MAC) of isoflurande with and without nitrous oxide in patients of various ages. Anesthesiology. 1975 Feb;42(2):197–200. doi: 10.1097/00000542-197502000-00014. [DOI] [PubMed] [Google Scholar]
- Steward A., Allott P. R., Cowles A. L., Mapleson W. W. Solubility coefficients for inhaled anaesthetics for water, oil and biological media. Br J Anaesth. 1973 Mar;45(3):282–293. doi: 10.1093/bja/45.3.282. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


