Abstract
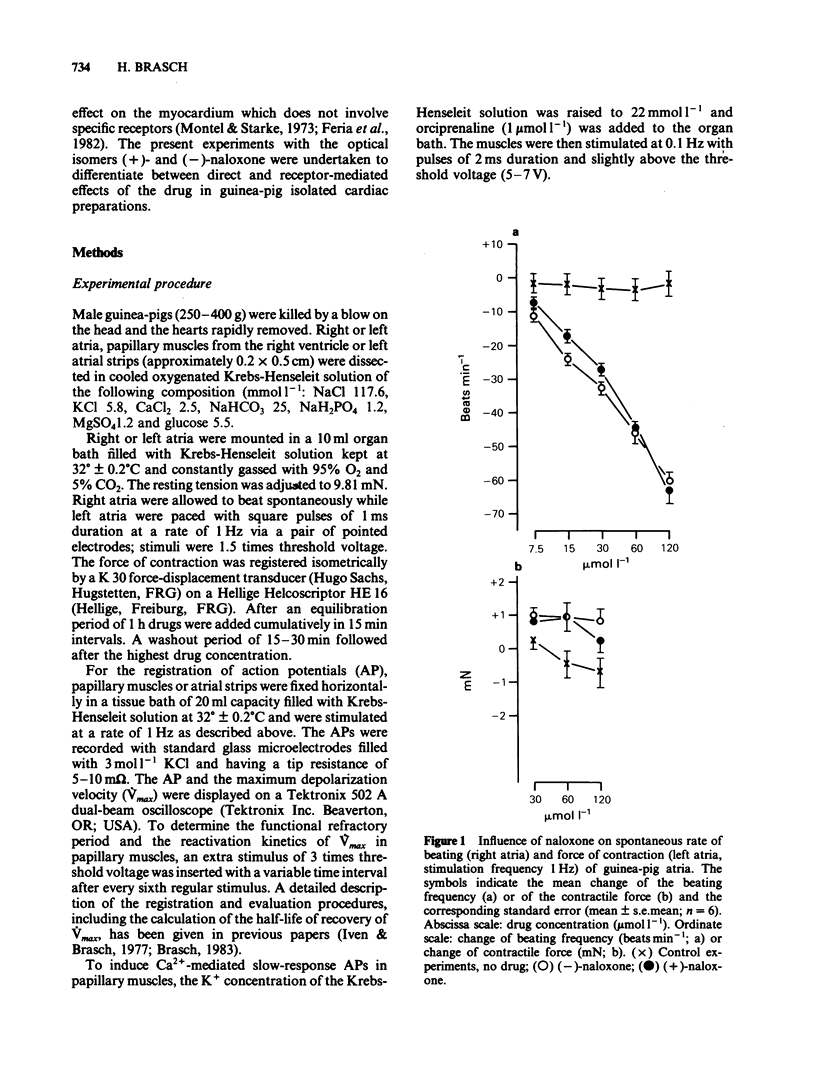
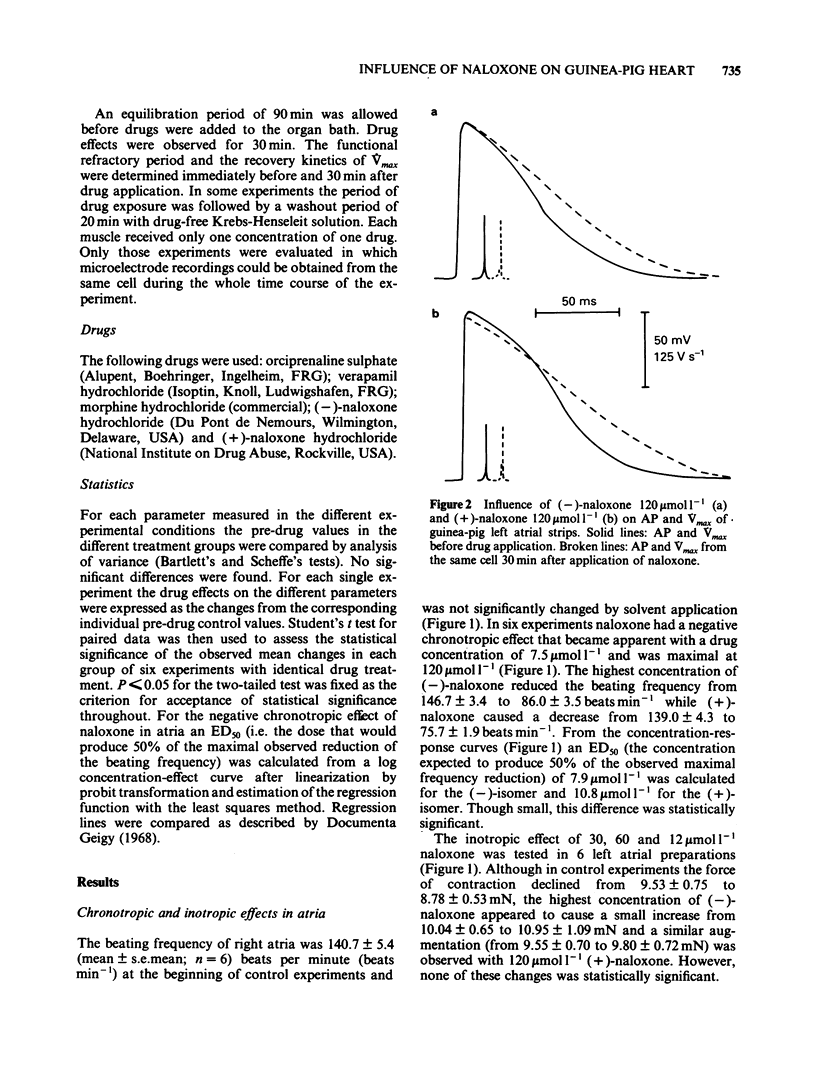
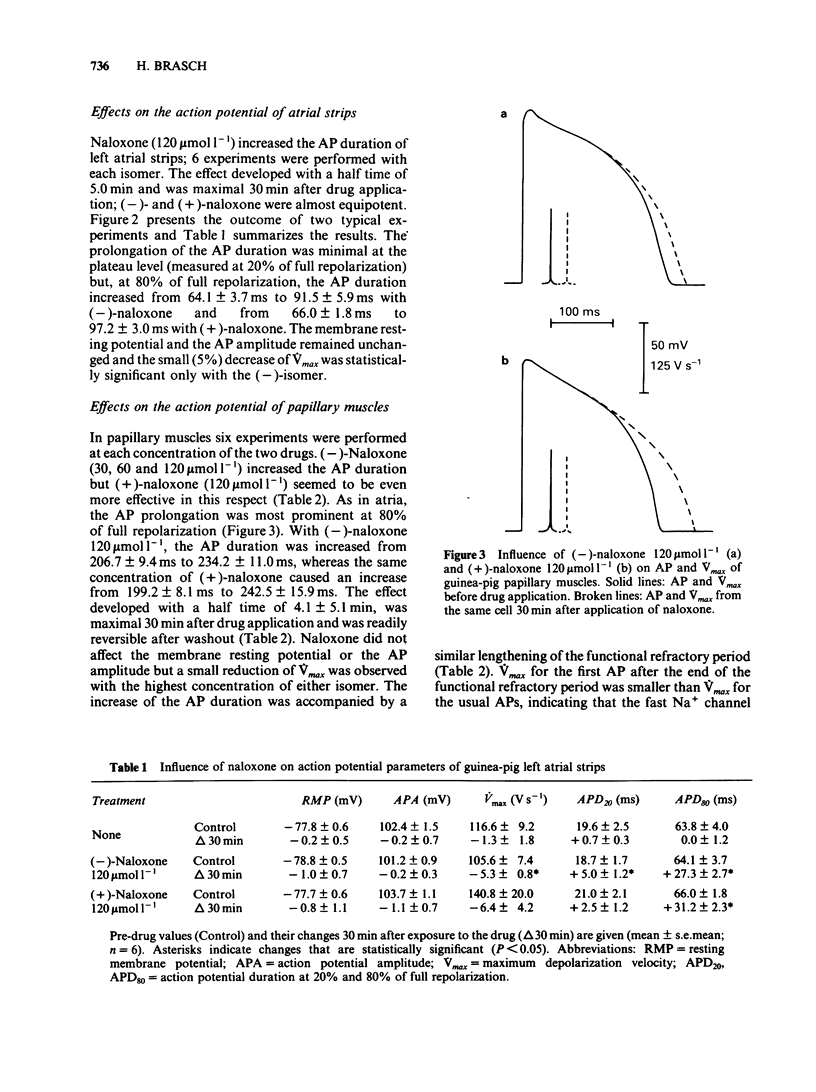
Naloxone in concentrations ranging from 7.5 to 120 mumol l-1 reduced the beating frequency of guinea-pig isolated atria. The ED50 was 7.9 mumol l-1 for the (-)-isomer and 10.8 mumol l-1 for the (+)-isomer. Concentrations up to 120 mumol l-1 of either (-)- or (+)-naloxone did not affect the force of contraction of left atria stimulated at 1 Hz. In concentrations from 30 to 120 mumol l-1 (-)-naloxone increased the action potential (AP) duration and the functional refractory period (FRP) of papillary muscles. The resting membrane potential and the AP amplitude remained unchanged, while a small decrease of Vmax was seen with the larger drug concentrations. The influence of (+)-naloxone (120 mumol l-1) was comparable to that of the (-)-isomer. The influence of morphine (120 mumol l-1) on papillary muscle AP was small. AP duration and FRP showed a marginal prolongation while Vmax was slightly decreased. (-)-Naloxone 60 mumol l-1 had no effect on slow-response APs of K+-depolarized papillary muscles. Slow-response APs were abolished by verapamil (1 mumol l-1). In left atrial strips the prolongation of the AP duration produced by 120 mumol l-1 of either (-)- or (+)-naloxone resembled the drug effect in papillary muscles. Most of the observed changes can be explained by an inhibition of the time-dependent membrane K+ outward current, an effect of naloxone that may be classified as a Class III antiarrhythmic action. Apparently this effect is not mediated by stereospecific opioid receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt J. O., Freye E. Opiate antagonist reverses the cardiovascular effects of inhalation anaesthesia. Nature. 1979 Feb 1;277(5695):399–400. doi: 10.1038/277399a0. [DOI] [PubMed] [Google Scholar]
- Ban T. Computer analysis of prolongation of cardiac action potential duration. Comput Biomed Res. 1983 Oct;16(5):403–413. doi: 10.1016/0010-4809(83)90030-7. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey J. L., Beil M. E. Antiarrhythmic evaluation of naloxone against acute coronary occlusion-induced arrhythmias in pigs. Eur J Pharmacol. 1983 Jun 17;90(4):427–431. doi: 10.1016/0014-2999(83)90566-6. [DOI] [PubMed] [Google Scholar]
- Brandt L., Andersson K. E., Hindfelt B., Ljunggren B., Pickard J. D. Are the vascular effects of naloxone attributable to the preservatives methyl- and propylparaben? J Cereb Blood Flow Metab. 1983 Sep;3(3):395–398. doi: 10.1038/jcbfm.1983.58. [DOI] [PubMed] [Google Scholar]
- Brasch H. Lack of direct antiarrhythmic electrophysiological effects of salicylate on isolated guinea-pig myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1983 Aug;323(4):343–349. doi: 10.1007/BF00512474. [DOI] [PubMed] [Google Scholar]
- Caldecourt M. A., Palmer T. N., Steare S. E., Sugden M. C., Watts D. I. Is the direct effect of naloxone on liver cell metabolism an artifact? Biochim Biophys Acta. 1983 Sep 13;759(3):303–305. doi: 10.1016/0304-4165(83)90328-8. [DOI] [PubMed] [Google Scholar]
- Carratu' M. R., Mitolo-Chieppa D. Inhibition of ionic currents in frog node of Ranvier treated with naloxone. Br J Pharmacol. 1982 Sep;77(1):115–119. doi: 10.1111/j.1476-5381.1982.tb09276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden L. E., Ruth J. A. Enkephalins modulate the responsiveness of rat atria in vitro to norepinephrine. Peptides. 1982 May-Jun;3(3):475–478. doi: 10.1016/0196-9781(82)90110-3. [DOI] [PubMed] [Google Scholar]
- Fagbemi O., Leprán I., Parratt J. R., Szekeres L. Naloxone inhibits early arrhythmias resulting from acute coronary ligation. Br J Pharmacol. 1982 Aug;76(4):504–506. doi: 10.1111/j.1476-5381.1982.tb09246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier D. T., Ohta M., Narahashi T. Nature of the morphine receptor present in the squid axon. Proc Soc Exp Biol Med. 1973 Apr;142(4):1209–1214. doi: 10.3181/00379727-142-37210. [DOI] [PubMed] [Google Scholar]
- Gautret B., Schmitt H. Cardiac slowing induced by peripheral kappa-opiate receptor stimulation in rats. Eur J Pharmacol. 1984 Jun 15;102(1):159–163. doi: 10.1016/0014-2999(84)90351-0. [DOI] [PubMed] [Google Scholar]
- Gayton R. J., Lambert L. A., Bradley P. B. Failure of (+)-naloxone to antagonise responses to opioid peptides. Neuropharmacology. 1978 Jul;17(7):549–551. doi: 10.1016/0028-3908(78)90063-1. [DOI] [PubMed] [Google Scholar]
- Holaday J. W. Cardiovascular effects of endogenous opiate systems. Annu Rev Pharmacol Toxicol. 1983;23:541–594. doi: 10.1146/annurev.pa.23.040183.002545. [DOI] [PubMed] [Google Scholar]
- Holaday J. W., D'Amato R. J., Ruvio B. A., Feuerstein G., Faden A. I. Adrenalectomy blocks pressor responses to naloxone in endotoxic shock: evidence for sympathomedullary involvement. Circ Shock. 1983;11(3):201–210. [PubMed] [Google Scholar]
- Iven H., Brasch H. Effects of the local anesthetics brufacain and lidocaine on transmembrane action potentials, refractory period, and reactivation of the sodium system in guinea pig heart muscle. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(2):153–161. doi: 10.1007/BF00499925. [DOI] [PubMed] [Google Scholar]
- Iven H., Zetler G. Failure of peptides (angiotensin, bradykinin, substance P, physalaemin, Met-enkephalin) to influence the cardiac action potential. Pharmacology. 1980;21(6):403–406. doi: 10.1159/000137461. [DOI] [PubMed] [Google Scholar]
- Lang R. E., Hermann K., Dietz R., Gaida W., Ganten D., Kraft K., Unger T. Evidence for the presence of enkephalins in the heart. Life Sci. 1983 Jan 24;32(4):399–406. doi: 10.1016/0024-3205(83)90086-3. [DOI] [PubMed] [Google Scholar]
- Ledda F., Mantelli L., Corti V., Fantozzi R. Inhibition of the cardiac response to sympathetic nerve stimulation by opioid peptides and its potentiation by morphine and methadone. Eur J Pharmacol. 1984 Jul 20;102(3-4):443–450. doi: 10.1016/0014-2999(84)90565-x. [DOI] [PubMed] [Google Scholar]
- Ledda F., Mantelli L. Possible presynaptic inhibitory effect of etorphine on sympathetic nerve terminals of guinea-pig heart. Eur J Pharmacol. 1982 Nov 19;85(2):247–250. doi: 10.1016/0014-2999(82)90476-9. [DOI] [PubMed] [Google Scholar]
- Montel H., Starke K. Effects of narcotic analgesics and their antagonists on the rabbit isolated heart and its adrenergic nerves. Br J Pharmacol. 1973 Dec;49(4):628–641. doi: 10.1111/j.1476-5381.1973.tb08538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHAN P. W., SEARS T. A. Action of methyl hydroxybenzoate on nervous conduction. Nature. 1961 Nov 18;192:668–669. doi: 10.1038/192668a0. [DOI] [PubMed] [Google Scholar]
- Ngai S. H., Berkowitz B. A., Yang J. C., Hempstead J., Spector S. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976 May;44(5):398–401. doi: 10.1097/00000542-197605000-00008. [DOI] [PubMed] [Google Scholar]
- Pace N. L., Parrish R. G., Lieberman M. M., Wong K. C., Blatnick R. A. Pharmacokinetics of naloxone and naltrexone in the dog. J Pharmacol Exp Ther. 1979 Feb;208(2):254–256. [PubMed] [Google Scholar]
- Pallasch T. J., Gill C. J. Naloxone-associated morbidity and mortality. Oral Surg Oral Med Oral Pathol. 1981 Dec;52(6):602–603. doi: 10.1016/0030-4220(81)90077-3. [DOI] [PubMed] [Google Scholar]
- Ruth J. A., Cuizon J. V., Eiden L. E. Leucine-enkephalin increases norepinephrine-stimulated chronotropy and 45Ca++ uptake in guinea-pig atria. Neuropeptides. 1984 May;4(3):185–191. doi: 10.1016/0143-4179(84)90099-4. [DOI] [PubMed] [Google Scholar]
- Ruth J. A., Eiden L. E. Leucine-enkephalin modulation of catecholamine positive chronotropy in rat atria is receptor-specific and calcium-dependent. Neuropeptides. 1984 Mar;4(2):101–108. doi: 10.1016/0143-4179(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Weihe E., McKnight A. T., Corbett A. D., Hartschuh W., Reinecke M., Kosterlitz H. W. Characterization of opioid peptides in guinea-pig heart and skin. Life Sci. 1983;33 (Suppl 1):711–714. doi: 10.1016/0024-3205(83)90601-x. [DOI] [PubMed] [Google Scholar]
- Williams E. M. Electrophysiological basis for a rational approach to antidysrhythmic drug therapy. Adv Drug Res. 1974;9:69–101. [PubMed] [Google Scholar]


