Abstract
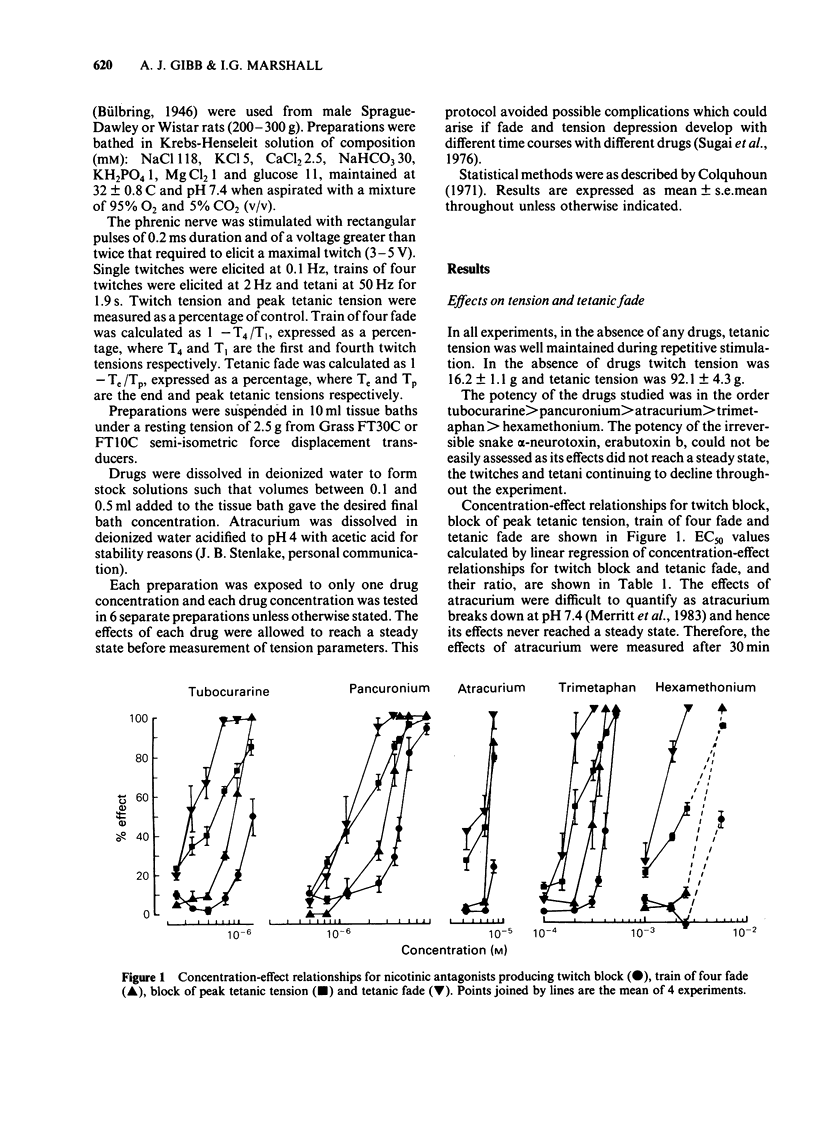
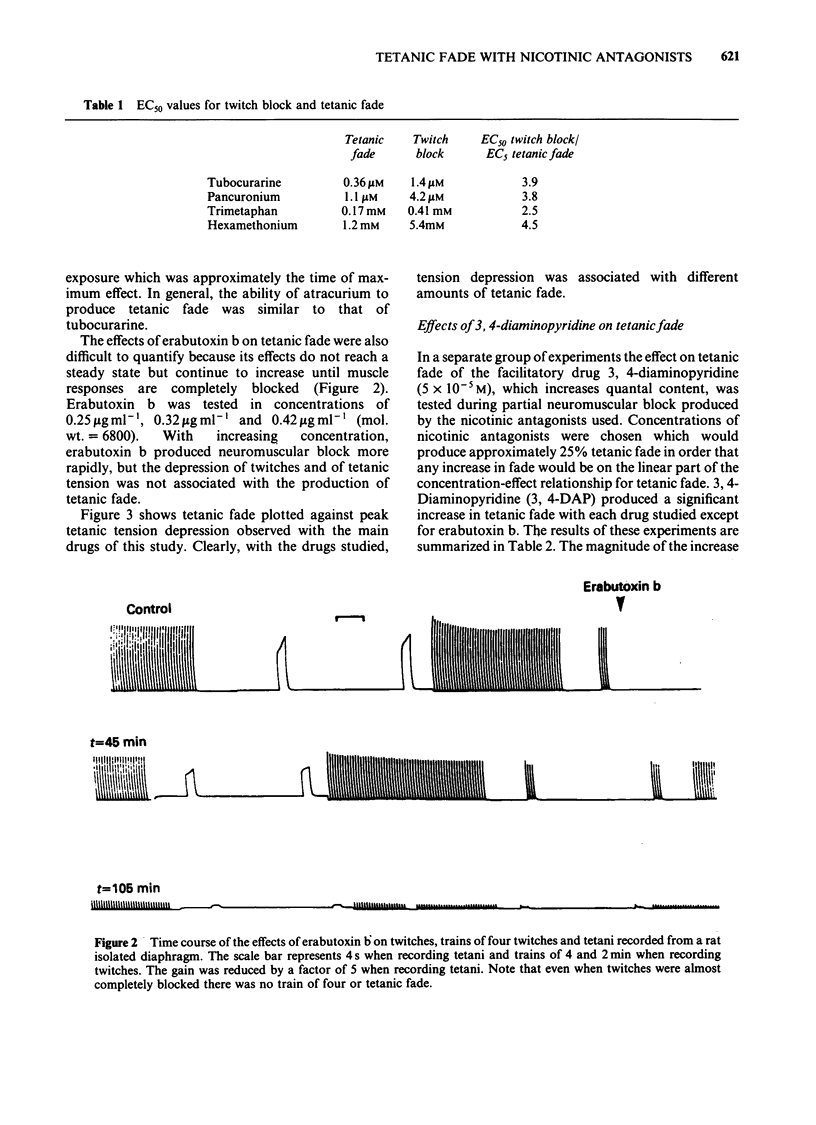
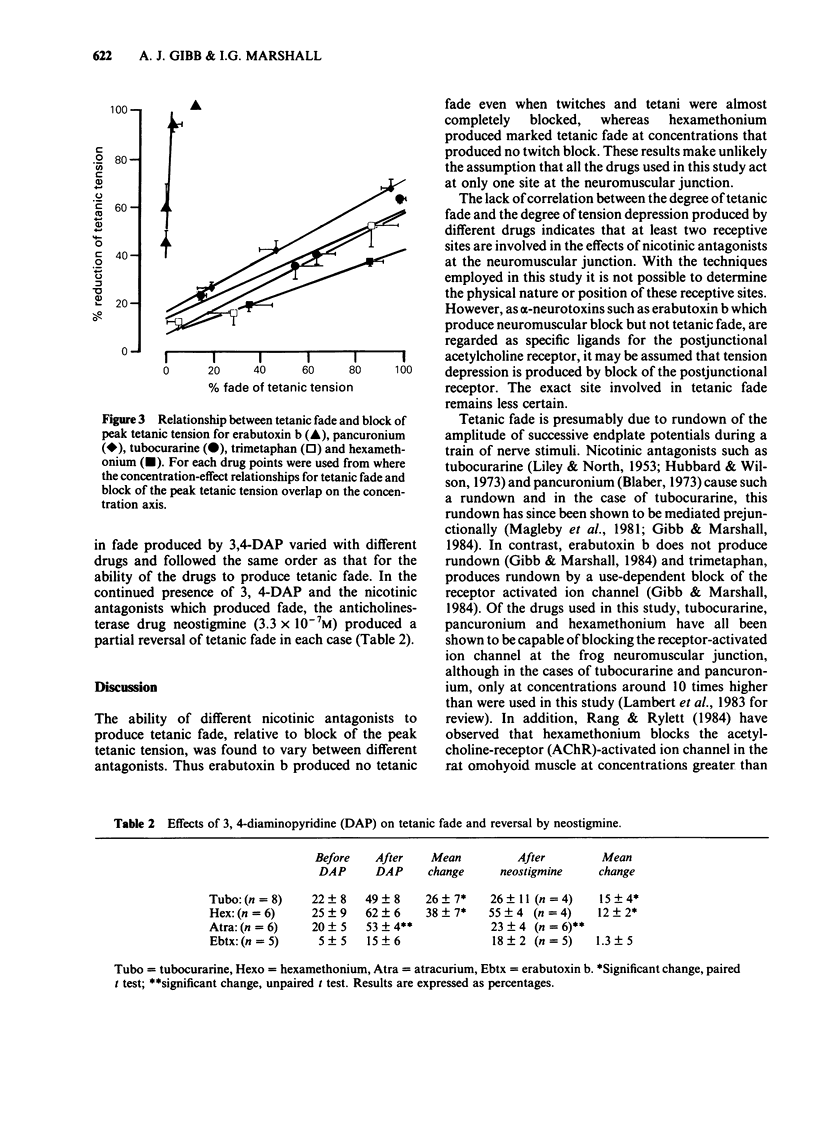
The effects of nicotine antagonists on single twitches, trains of four twitches and tetanic contractions of the isolated diaphragm of the rat were examined. Different drugs were found to produce different amounts of tetanic fade relative to depression of twitch tension. The order of activity from most able, to least able to produce fade was: hexamethonium greater than trimetaphan=atracurium=tubocurarine greater than pancuronium greater than erabutoxin b. The effect of erabutoxin b was distinctive for its almost complete lack of tetanic fade. 3,4-Diaminopyridine increased tetanic fade produced by tubocurarine, atracurium and hexamethonium, but not that produced by erabutoxin b. It is concluded that nicotinic antagonists act at more than one site at the neuromuscular junction. Assuming block of the postjunctional acetylcholine receptor produces tension depression, a second or third site must be involved in producing tetanic fade. The possibility that tetanic fade results from block of the ion channel associated with the postjunctional acetylcholine receptor or from the block of a prejunctional nicotinic receptor is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaber L. C. The prejunctional actions of some non-depolarizing blocking drugs. Br J Pharmacol. 1973 Jan;47(1):109–116. doi: 10.1111/j.1476-5381.1973.tb08163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C. Prejunctional and postjunctional cholinoceptors at the neuromuscular junction. Anesth Analg. 1980 Dec;59(12):935–943. [PubMed] [Google Scholar]
- Bowman W. C., Webb S. N. Tetanic fade during partial transmission failure produced by non-depolarizing neuromuscular blocking drugs in the cat. Clin Exp Pharmacol Physiol. 1976 Nov-Dec;3(6):545–555. doi: 10.1111/j.1440-1681.1976.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Marshall I. G. Pre-and post-junctional effects of tubocurarine and other nicotinic antagonists during repetitive stimulation in the rat. J Physiol. 1984 Jun;351:275–297. doi: 10.1113/jphysiol.1984.sp015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F. Post-tetanic restoration of neuromuscular transmission blocked by D-tubocurarine. J Physiol. 1952 Oct;118(2):216–227. doi: 10.1113/jphysiol.1952.sp004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Durant N. N., Henderson E. G. Drug-induced modification of ionic conductance at the neuromuscular junction. Annu Rev Pharmacol Toxicol. 1983;23:505–539. doi: 10.1146/annurev.pa.23.040183.002445. [DOI] [PubMed] [Google Scholar]
- Lee C., Chen D., Katz R. L. Characteristics of nondepolarizing neuromuscular block: (I) post-junctional block by alpha-bungarotoxin. Can Anaesth Soc J. 1977 Mar;24(2):212–219. doi: 10.1007/BF03006234. [DOI] [PubMed] [Google Scholar]
- Merrett R. A., Thompson C. W., Webb F. W. In vitro degradation of atracurium in human plasma. Br J Anaesth. 1983 Jan;55(1):61–66. doi: 10.1093/bja/55.1.61. [DOI] [PubMed] [Google Scholar]
- PATON W. D. M., PERRY W. L. M. The pharmacology of the toxiferines. Br J Pharmacol Chemother. 1951 Jun;6(2):299–310. doi: 10.1111/j.1476-5381.1951.tb00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. The methonium. Pharmacol Rev. 1952 Sep;4(3):219–253. [PubMed] [Google Scholar]
- Paton W. D., Waud D. R. The margin of safety of neuromuscular transmission. J Physiol. 1967 Jul;191(1):59–90. doi: 10.1113/jphysiol.1967.sp008237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Rylett R. J. The interaction between hexamethonium and tubocurarine on the rat neuromuscular junction. Br J Pharmacol. 1984 Mar;81(3):519–531. doi: 10.1111/j.1476-5381.1984.tb10105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai N., Hughes R., Payne J. P. Sequential changes in the fade of tetanic tension after the administration of tubocurarine in anaesthetized man. Br J Anaesth. 1976 Jun;48(6):535–539. doi: 10.1093/bja/48.6.535. [DOI] [PubMed] [Google Scholar]
- Thomsen R. H., Wilson D. F. Effects of 4-aminopyridine and 3,4-diaminopyridine on transmitter release at the neuromuscular junction. J Pharmacol Exp Ther. 1983 Oct;227(1):260–265. [PubMed] [Google Scholar]
- Williams N. E., Webb S. N., Calvey T. N. Differential effects of myoneural blocking drugs on neuromuscular transmission. Br J Anaesth. 1980 Nov;52(11):1111–1115. doi: 10.1093/bja/52.11.1111. [DOI] [PubMed] [Google Scholar]
- Wilson D. F. Influence of presynaptic receptors on neuromuscular transmission in rat. Am J Physiol. 1982 May;242(5):C366–C372. doi: 10.1152/ajpcell.1982.242.5.C366. [DOI] [PubMed] [Google Scholar]


