Abstract
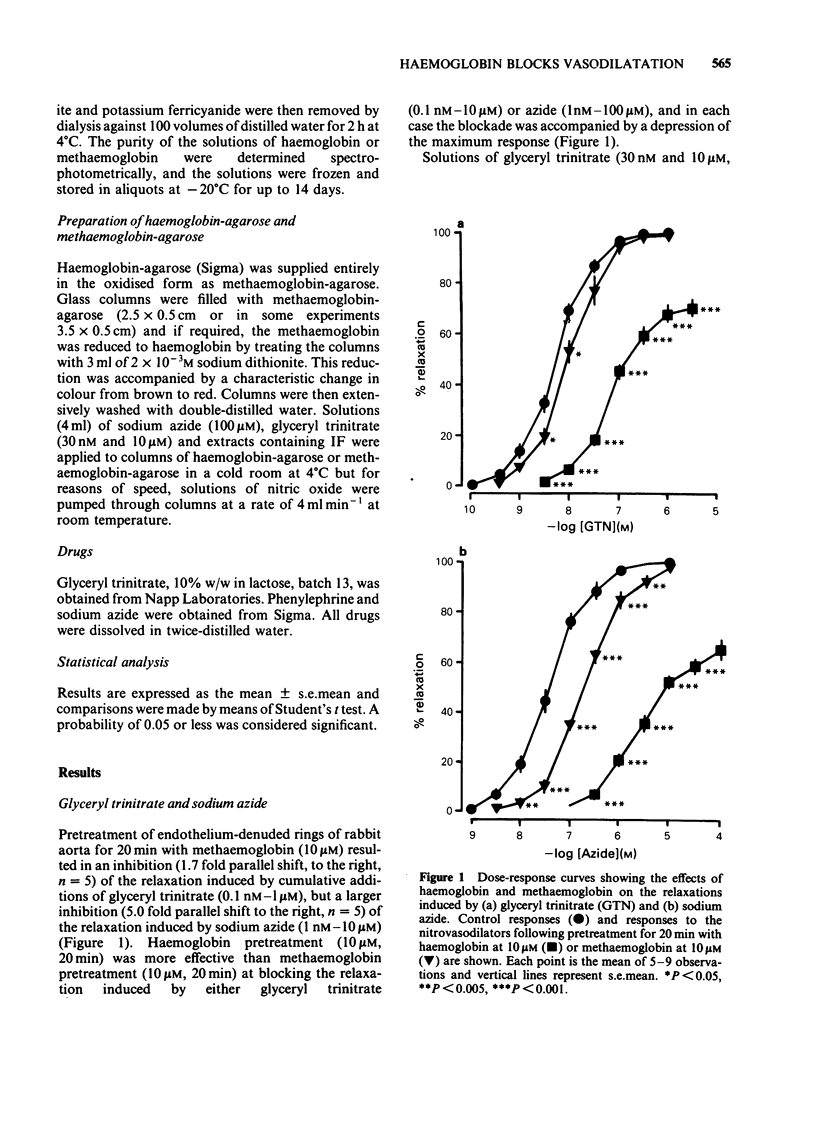
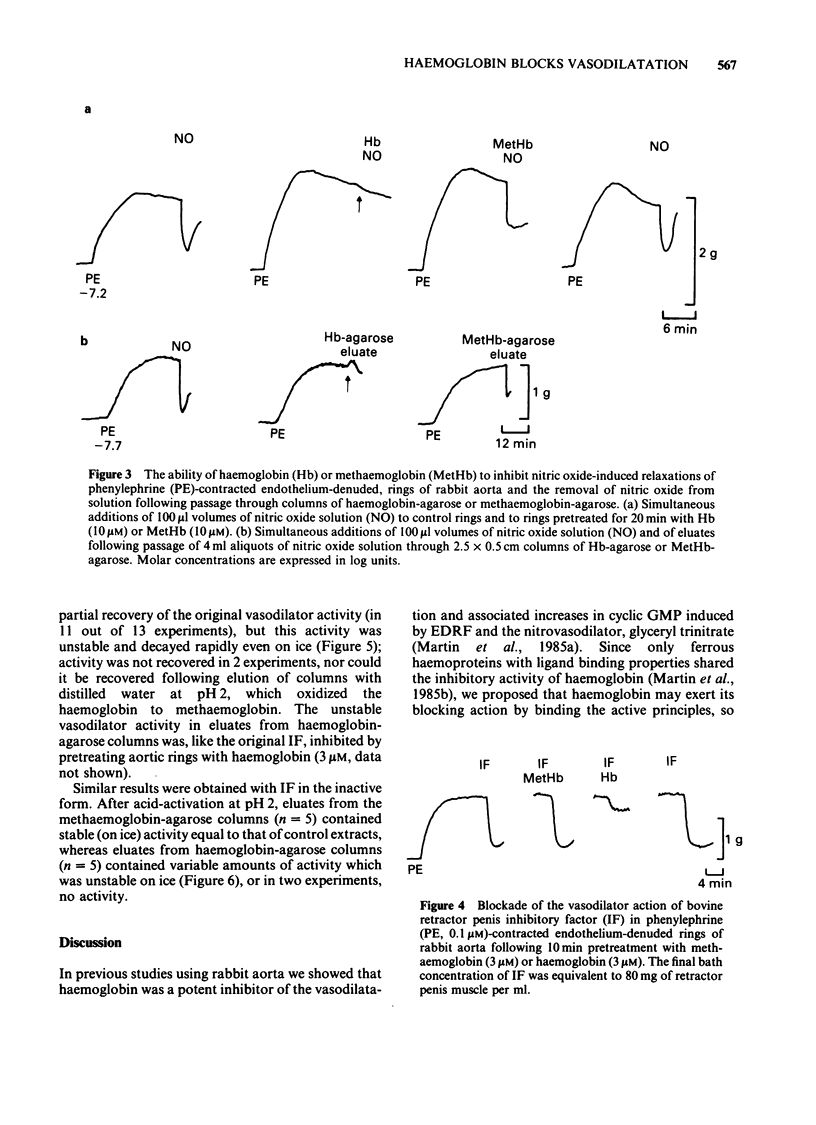
The mechanisms by which haemoglobin and methaemoglobin inhibit the vasodilator actions of glyceryl trinitrate, sodium azide, nitric oxide, and the bovine retractor penis inhibitory factor (IF) were studied on rabbit endothelium-denuded aortic rings. Methaemoglobin was less effective than haemoglobin against each vasodilator, it was more effective at inhibiting the relaxation to azide than that to glyceryl trinitrate. Glyceryl trinitrate was neither bound nor inactivated when passed through columns of haemoglobin-agarose or methaemoglobin-agarose. Azide was reversibly bound but less by haemoglobin-agarose than by methaemoglobin-agarose. Inhibition of the vasodilator actions of glyceryl trinitrate is not attributable therefore to a direct interaction with the haemoproteins, although a small part of the inhibition of azide-induced relaxation by methaemoglobin is likely to be due to a direct interaction. Columns of haemoglobin-agarose were more effective than columns of methaemoglobin-agarose in removing nitric oxide from solution. The greater ability of haemoglobin, compared to methaemoglobin, to inhibit vasodilatation induced by nitrovasodilators may therefore reflect the greater ability of haemoglobin to bind nitric oxide which is the active principle of the nitrovasodilators. Neither the acid-activated nor the inactive forms of IF were bound or inactivated when passed through columns of methaemoglobin-agarose. Neither form of IF was retained on passage through columns of haemoglobin-agarose, but the resulting activity in the eluates was less than control, was unstable and, unlike the original activity, decayed rapidly on ice.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Killick S. W., Aboo Aar M. Extraction from ox retractor penis of an inhibitory substance which mimics its atropine-resistant neurogenic relaxation. Br J Pharmacol. 1975 Jul;54(3):409–410. doi: 10.1111/j.1476-5381.1975.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem. 1978 Dec 10;253(23):8433–8443. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957 May 23;136(3):507–524. doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzer R., Böhme E., Hofmann F., Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981 Sep 14;132(1):71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Hofmann F., Schultz G. Purification of a soluble, sodium-nitroprusside-stimulated guanylate cyclase from bovine lung. Eur J Biochem. 1981 Jun 1;116(3):479–486. doi: 10.1111/j.1432-1033.1981.tb05361.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Hunter J. C., Martin W. Some physical and chemical properties of the smooth muscle inhibitory factor in extracts of the bovine retractor penis muscle. J Physiol. 1981 Jun;315:111–125. doi: 10.1113/jphysiol.1981.sp013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Kadowitz P. J., Ignarro L. J. Coronary arterial relaxation and guanylate cyclase activation by cigarette smoke, N'-nitrosonornicotine and nitric oxide. J Pharmacol Exp Ther. 1980 Jul;214(1):9–15. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. J Pharmacol Exp Ther. 1985 Jun;233(3):679–685. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Miki N., Kawabe Y., Kuriyama K. Activation of cerebral guanylate cyclase by nitric oxide. Biochem Biophys Res Commun. 1977 Apr 25;75(4):851–856. doi: 10.1016/0006-291x(77)91460-7. [DOI] [PubMed] [Google Scholar]
- Mittal C. K., Kimura H., Murad F. Requirement for a macromolecular factor for sodium azide activation of guanulate cyclase. J Cyclic Nucleotide Res. 1975;1(6):261–269. [PubMed] [Google Scholar]
- Ohlstein E. H., Wood K. S., Ignarro L. J. Purification and properties of heme-deficient hepatic soluble guanylate cyclase: effects of heme and other factors on enzyme activation by NO, NO-heme, and protoporphyrin IX. Arch Biochem Biophys. 1982 Oct 1;218(1):187–198. doi: 10.1016/0003-9861(82)90335-6. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent vasodilator-and nitrovasodilator-induced relaxation may be mediated through cyclic GMP formation and cyclic GMP-dependent protein phosphorylation. Trans Assoc Am Physicians. 1983;96:19–30. [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Schultz K., Schultz K., Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977 Feb 24;265(5596):750–751. doi: 10.1038/265750a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Manganiello V. C., Vaughan M. Regulation of activity of purified guanylate cyclase from liver that is unresponsive to nitric oxide. Biochem J. 1983 Dec 1;215(3):447–455. doi: 10.1042/bj2150447. [DOI] [PMC free article] [PubMed] [Google Scholar]


