Abstract
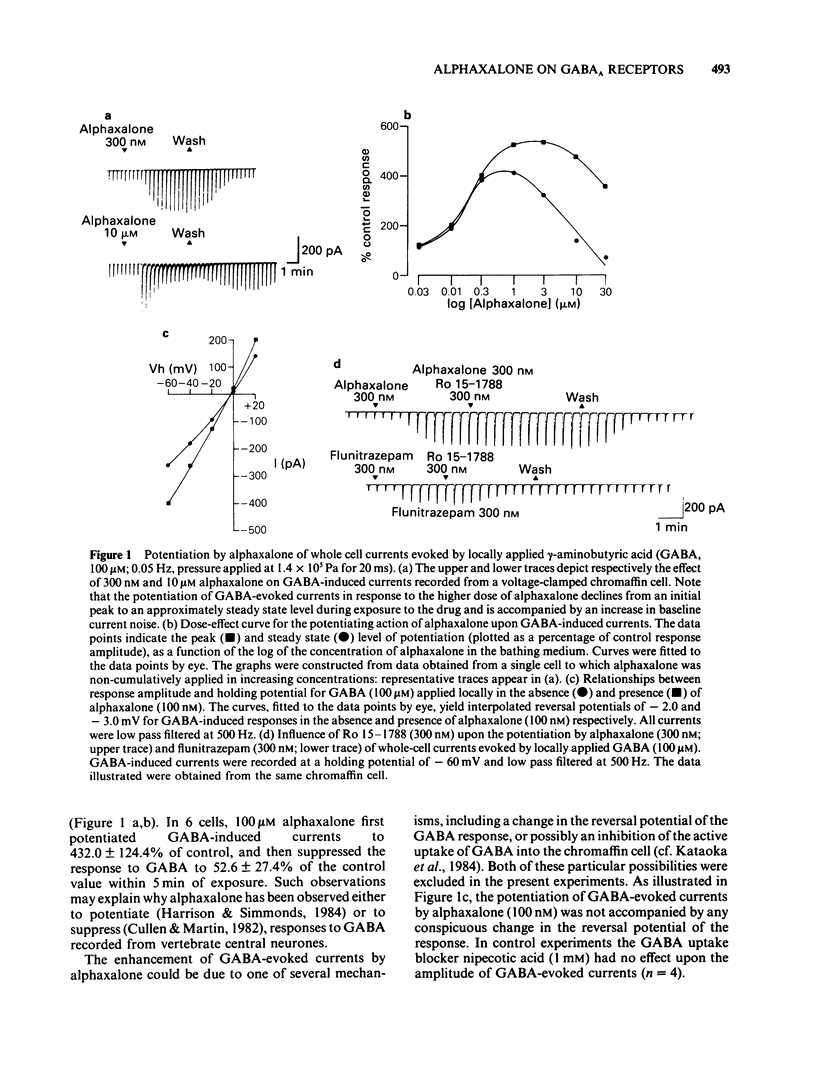
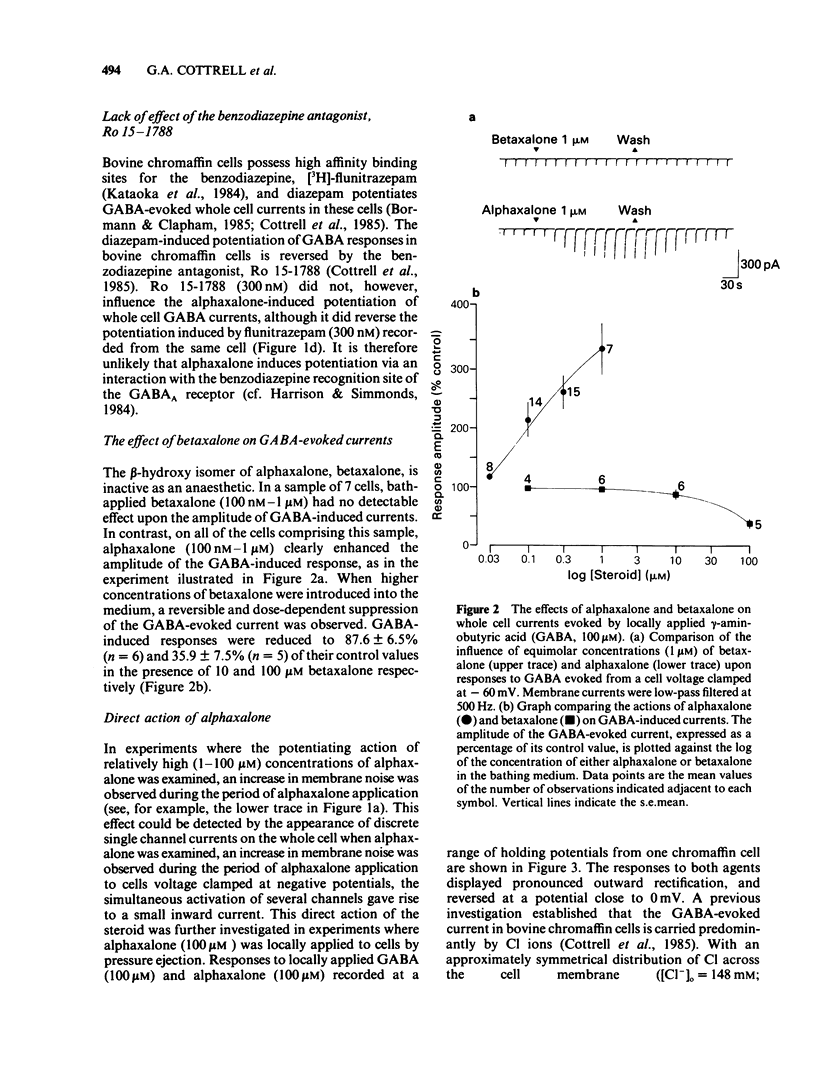
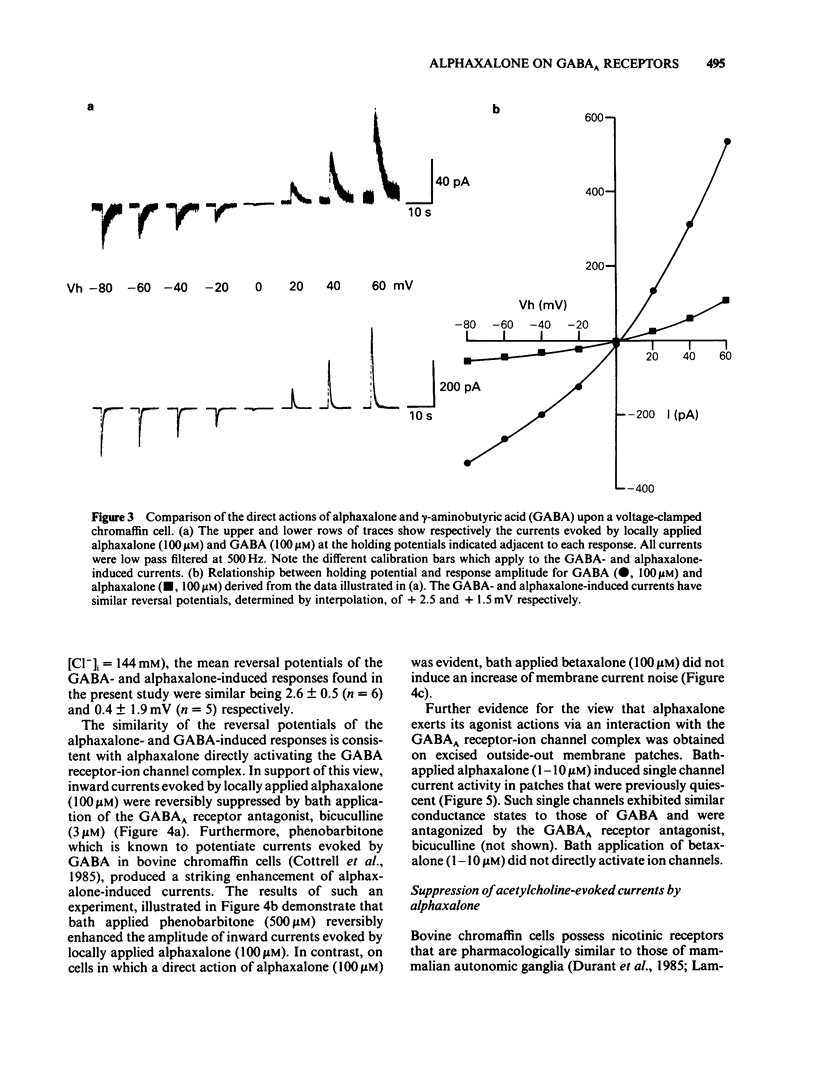
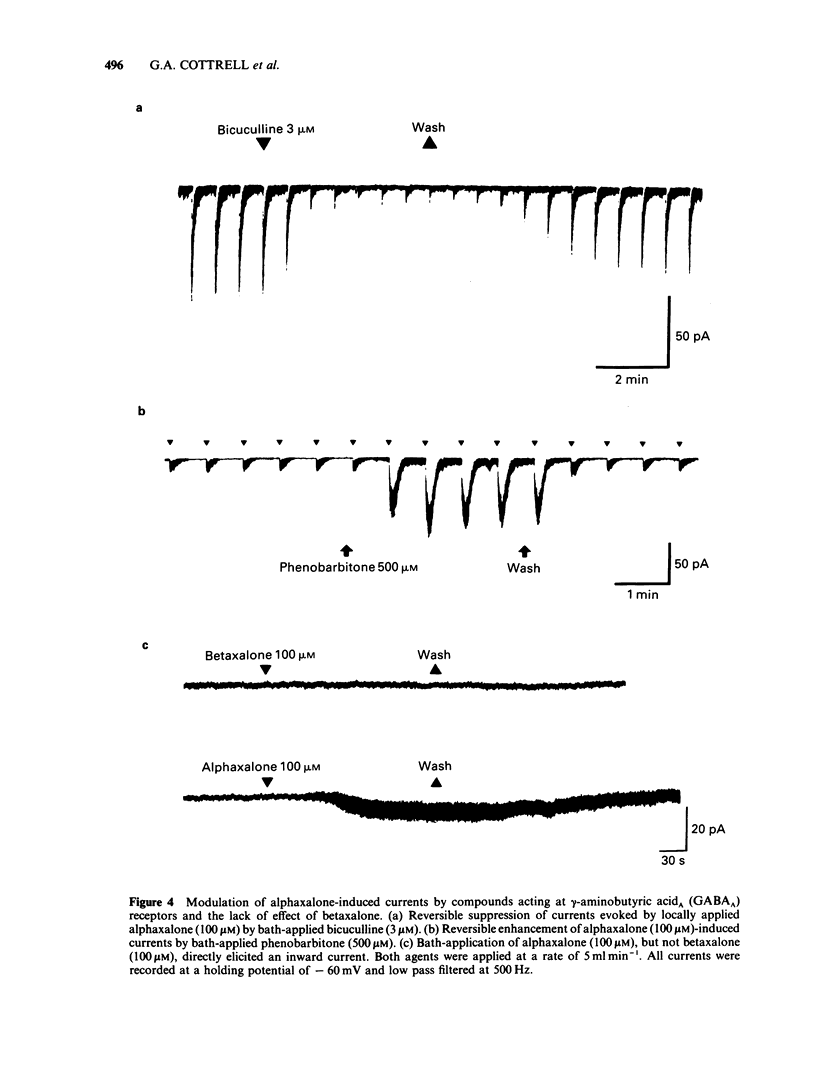
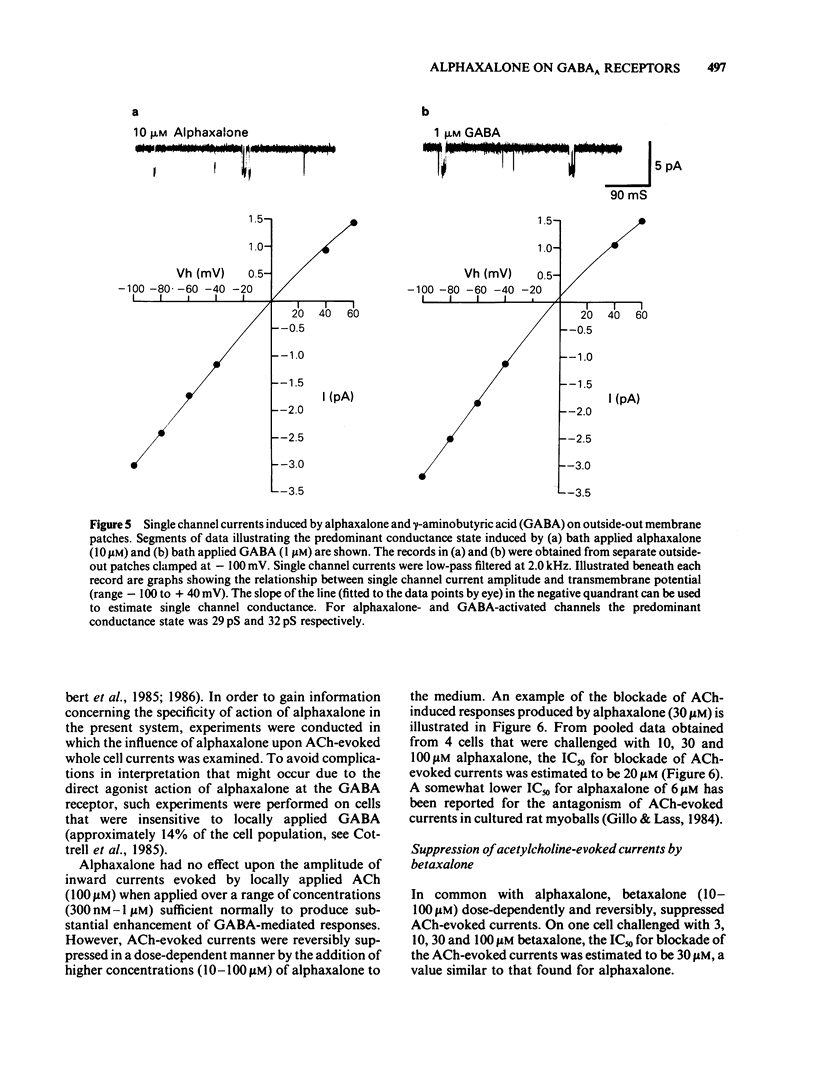
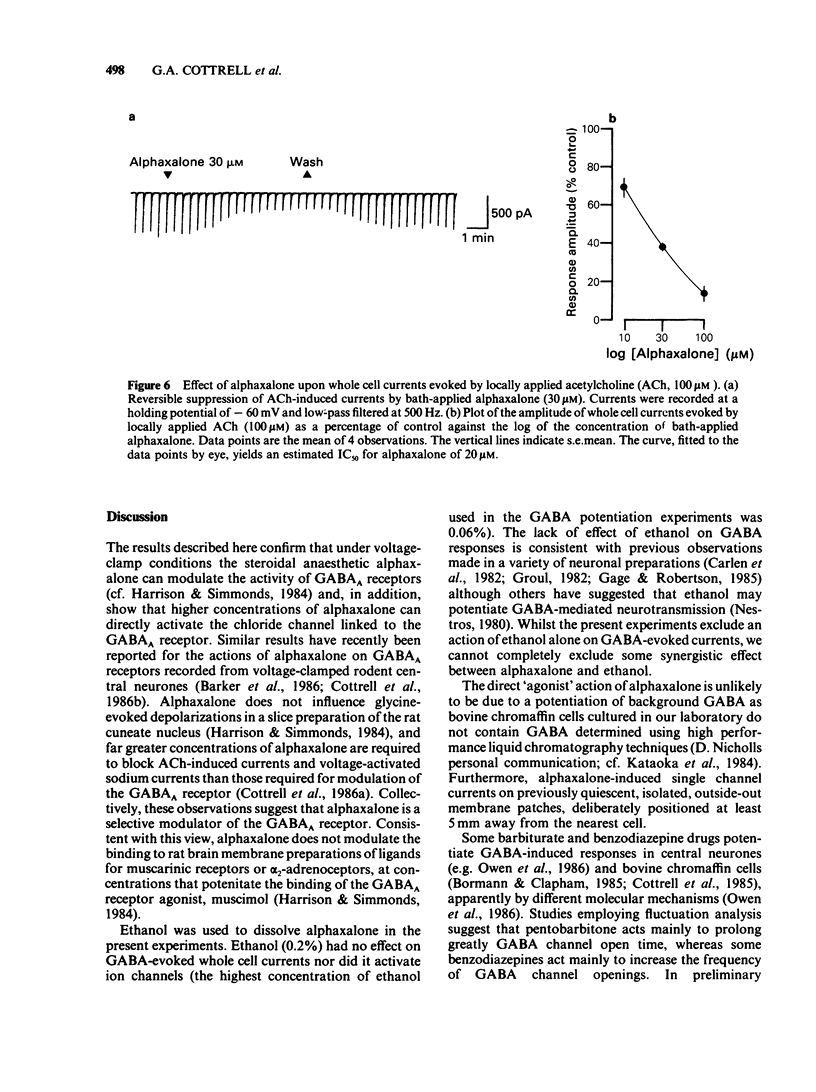
The modulation of the gamma-aminobutyric acidA (GABAA) receptor by alphaxalone has been investigated by use of voltage-clamp recordings from enzymatically isolated bovine chromaffin cells maintained in cell culture. Alphaxalone (greater than 30 nM) reversibly and dose-dependently potentiated the amplitude of membrane currents elicited by locally applied GABA (100 microM). The potentiation was not associated with a change in the reversal potential of GABA-evoked currents and was not influenced by the benzodiazepine receptor antagonist, Ro15-1788 (300 nM). At relatively high concentrations (greater than 1 microM), alphaxalone directly elicited a membrane current. It is concluded that such currents result from GABAA receptor activation since they were reversibly suppressed by bicuculline (3 microM), dose-dependently enhanced by phenobarbitone (100-500 microM), and had a similar reversal potential (approximately 0 mV) to currents elicited by GABA. Additionally, on outside-out membrane patches, alphaxalone activated single channel currents with amplitudes and a reversal potential similar to those evoked by GABA. Alphaxalone (30 nM-1 microM) had no effect upon the amplitude of membrane currents elicited by locally applied acetylcholine (ACh) (100 microM). However, higher concentrations of alphaxalone (10-100 microM) reversibly suppressed ACh-evoked currents, the IC50 for blockade being 20 microM. The beta-hydroxy isomer of alphaxalone, betaxalone (100 nM-1 microM), did not potentiate GABA-induced currents, nor did higher concentrations of the steroid (10-100 microM) directly evoke a membrane current. However, over the latter concentration range, betaxalone suppressed the amplitude of currents elicited either by GABA or ACh. The relevance of the present results to the anaesthetic action of alphaxalone is discussed together with the broader implications of steroidal modulation of the GABAA receptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. W., Shankley N. P. Pharmacological analysis of the muscarinic receptors involved when McN-A 343 stimulates acid secretion in the mouse isolated stomach. Br J Pharmacol. 1985 Nov;86(3):609–617. doi: 10.1111/j.1476-5381.1985.tb08937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Durand D. Ethanol in low doses augments calcium-mediated mechanisms measured intracellularly in hippocampal neurons. Science. 1982 Jan 15;215(4530):306–309. doi: 10.1126/science.7053581. [DOI] [PubMed] [Google Scholar]
- Child K. J., Currie J. P., Dis B., Dodds M. G., Pearce D. R., Twissell D. J. The pharmacological properties in animals of CT1341--a new steroid anaesthetic agent. Br J Anaesth. 1971 Jan;43(1):2–13. doi: 10.1093/bja/43.1.2-a. [DOI] [PubMed] [Google Scholar]
- Cullen K. D., Martin R. J. Dissimilar influences of some injectable anaesthetics on the responses of reticulo-spinal neurones to inhibitory transmitters in the lamprey. Br J Pharmacol. 1982 Nov;77(3):493–504. doi: 10.1111/j.1476-5381.1982.tb09323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillo B., Lass Y. The mechanism of steroid anaesthetic (alphaxalone) block of acetylcholine-induced ionic currents. Br J Pharmacol. 1984 Aug;82(4):783–789. doi: 10.1111/j.1476-5381.1984.tb16474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D. L. Ethanol alters synaptic activity in cultured spinal cord neurons. Brain Res. 1982 Jul 8;243(1):25–33. doi: 10.1016/0006-8993(82)91117-9. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984 Dec 10;323(2):287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Kataoka Y., Gutman Y., Guidotti A., Panula P., Wroblewski J., Cosenza-Murphy D., Wu J. Y., Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestoros J. N. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science. 1980 Aug 8;209(4457):708–710. doi: 10.1126/science.7394531. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch. 1980 Feb;383(3):249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Sear J. W., Prys-Roberts C. Plasma concentrations of alphaxalone during continuous infusion of Althesin. Br J Anaesth. 1979 Sep;51(9):861–865. doi: 10.1093/bja/51.9.861. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Brastrup C. Benzodiazepine receptors in rat brain. Nature. 1977 Apr 21;266(5604):732–734. doi: 10.1038/266732a0. [DOI] [PubMed] [Google Scholar]


