Abstract
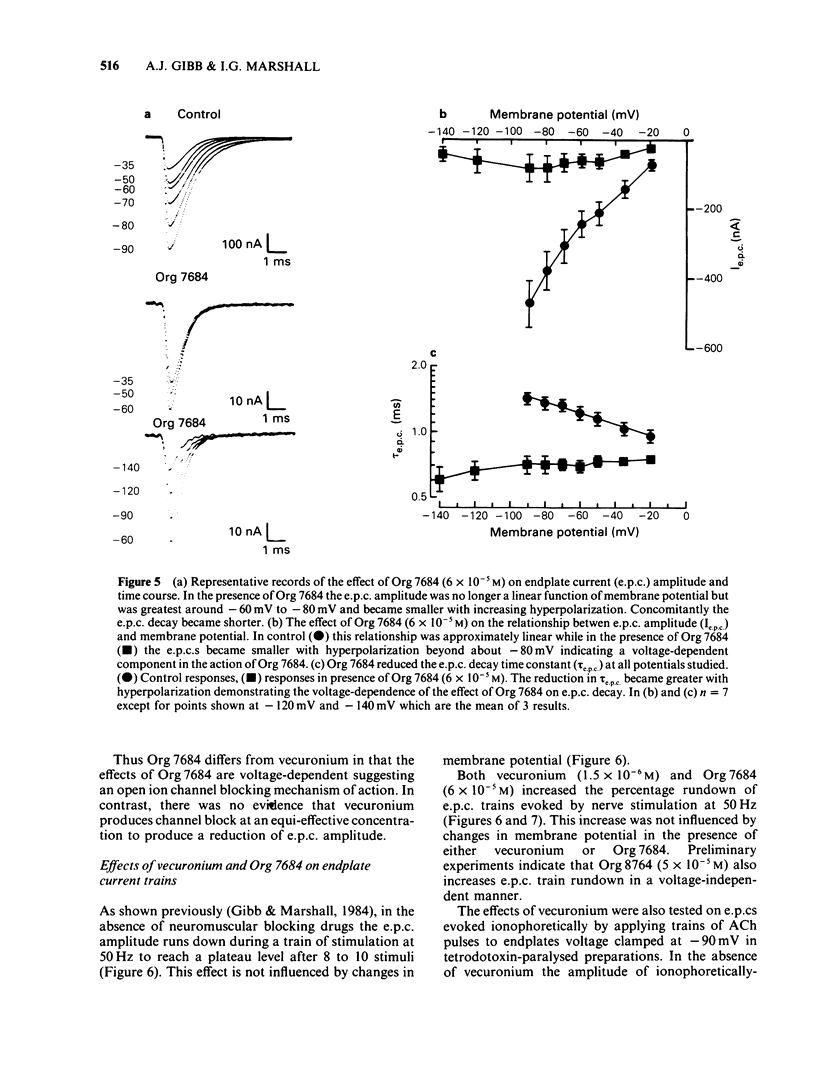
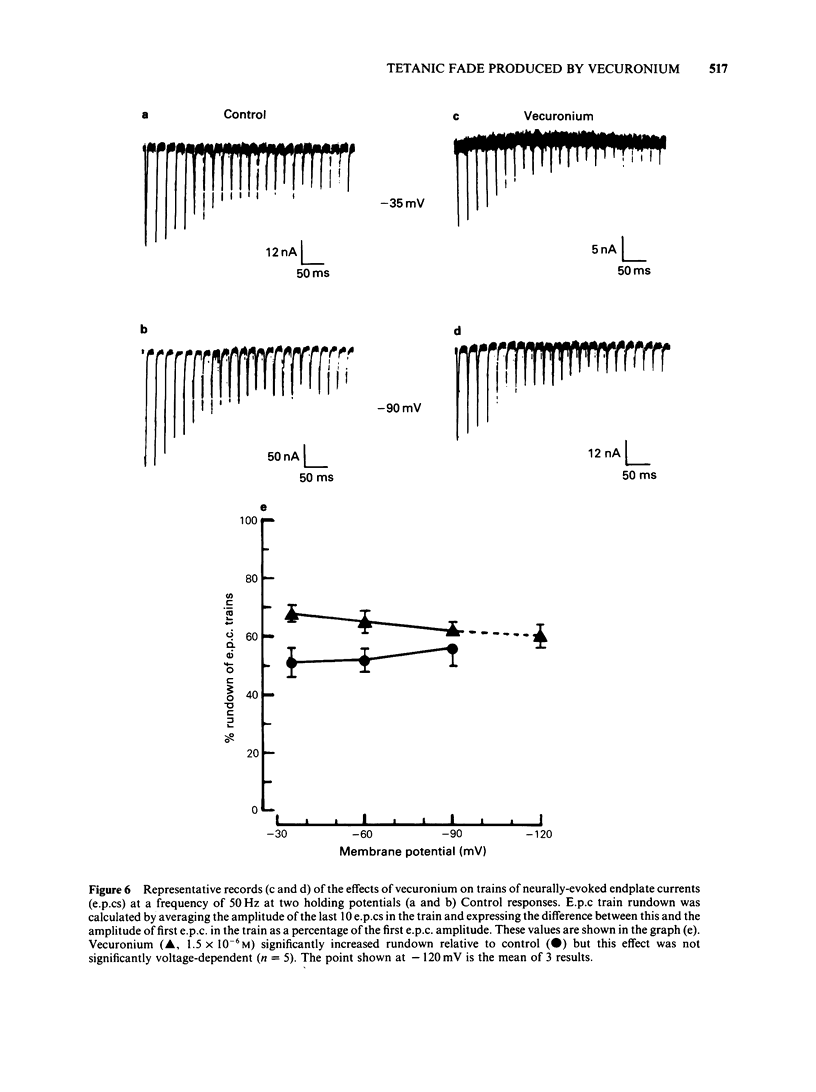
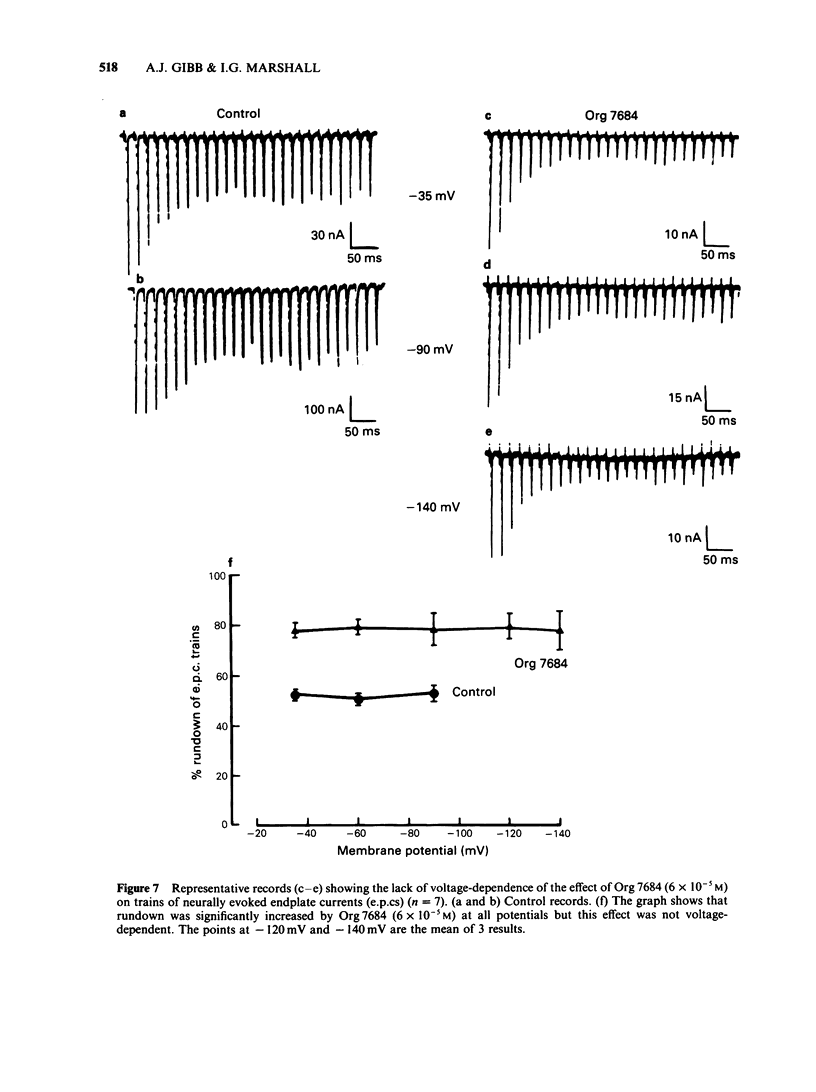
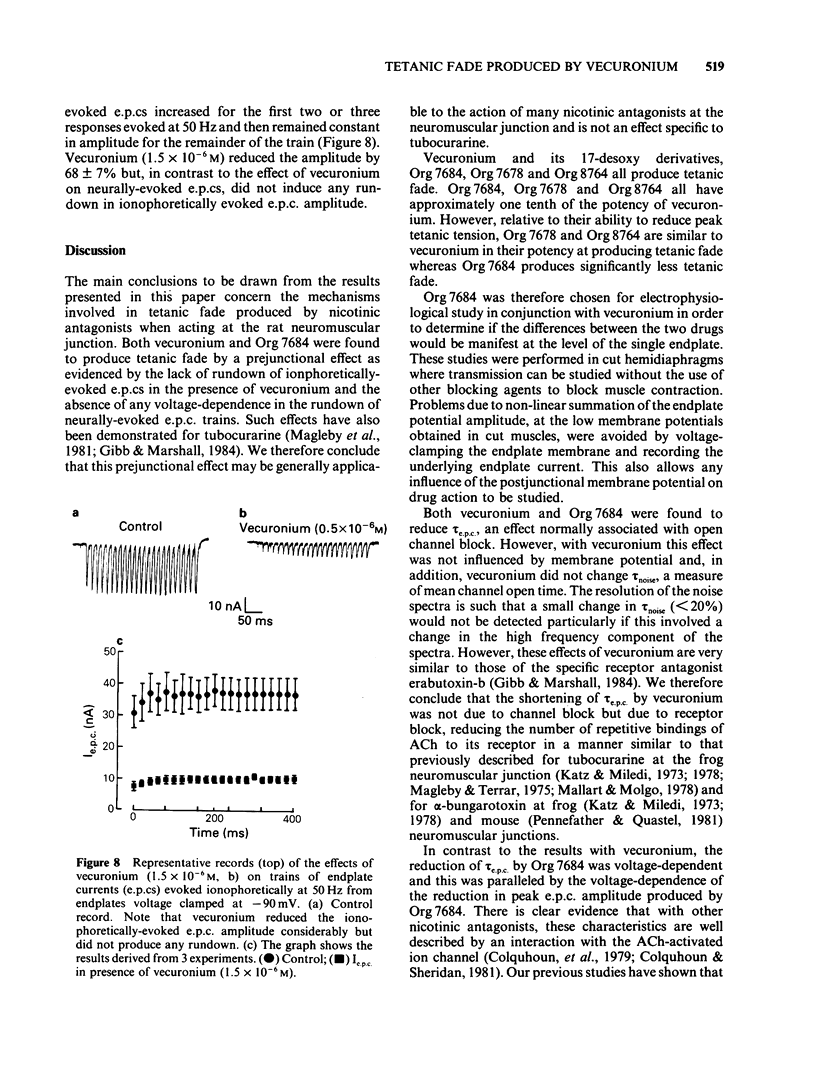
The effects of vecuronium (Org NC45), Org 7678 and Org 7684 were examined on twitches and tetani recorded from rat isolated diaphragms. Org 7678 and Org 7684 exhibited approximately one tenth of the neuromuscular blocking potency of vecuronium. At concentrations producing equivalent amounts of twitch block, Org 7684 produced significantly less tetanic fade than did vecuronium or Org 7678. In cut muscles both vecuronium and Org 7684 reduced the endplate current (e.p.c.) amplitude (Ie.p.c.), reduced e.p.c. decay time constant (tau e.p.c.), and increased the e.p.c. train rundown. The effects of vecuronium were not voltage-dependent and vecuronium did not change tau noise. The effect of Org 7684 on Ie.p.c. and tau e.p.c. became greater with hyperpolarization, but the effect on e.p.c. train rundown was not voltage-dependent. It is concluded that both vecuronium and Org 7684 produce e.p.c. train rundown and tetanic fade by a prejunctional mechanism. However, whereas postjunctionally vecuronium blocks only the acetylcholine receptor, Org 7684 blocks both the receptor and its associated ion channel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman W. C. Prejunctional and postjunctional cholinoceptors at the neuromuscular junction. Anesth Analg. 1980 Dec;59(12):935–943. [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The modes of action of gallamine. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):181–203. doi: 10.1098/rspb.1981.0002. [DOI] [PubMed] [Google Scholar]
- Dreyer F. Acetylcholine receptor. Br J Anaesth. 1982 Feb;54(2):115–130. doi: 10.1093/bja/54.2.115. [DOI] [PubMed] [Google Scholar]
- Durant N. N., Horn R. Org. 6368: a nondepolarizing neuromuscular blocking agent with a novel effect on end-plate conductance. J Pharmacol Exp Ther. 1984 Mar;228(3):567–572. [PubMed] [Google Scholar]
- Gibb A. J., Marshall I. G. Pre-and post-junctional effects of tubocurarine and other nicotinic antagonists during repetitive stimulation in the rat. J Physiol. 1984 Jun;351:275–297. doi: 10.1113/jphysiol.1984.sp015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. J., Durant N. N., Henderson E. G. Drug-induced modification of ionic conductance at the neuromuscular junction. Annu Rev Pharmacol Toxicol. 1983;23:505–539. doi: 10.1146/annurev.pa.23.040183.002445. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S., Terrar D. A. The effect of (+)-tubocurarine on neuromuscular transmission during repetitive stimulation in the rat, mouse, and frog. J Physiol. 1981 Mar;312:97–113. doi: 10.1113/jphysiol.1981.sp013618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Molgó J. The effects of pH and curare on the time course of end-plate currents at the neuromuscular junction of the frog. J Physiol. 1978 Mar;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Relation between subsynaptic receptor blockade and response to quantal transmitter at the mouse neuromuscular junction. J Gen Physiol. 1981 Sep;78(3):313–344. doi: 10.1085/jgp.78.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert F. G. Release of transmitter at the neuromuscular junction. Br J Anaesth. 1982 Feb;54(2):131–145. doi: 10.1093/bja/54.2.131. [DOI] [PubMed] [Google Scholar]


