Abstract
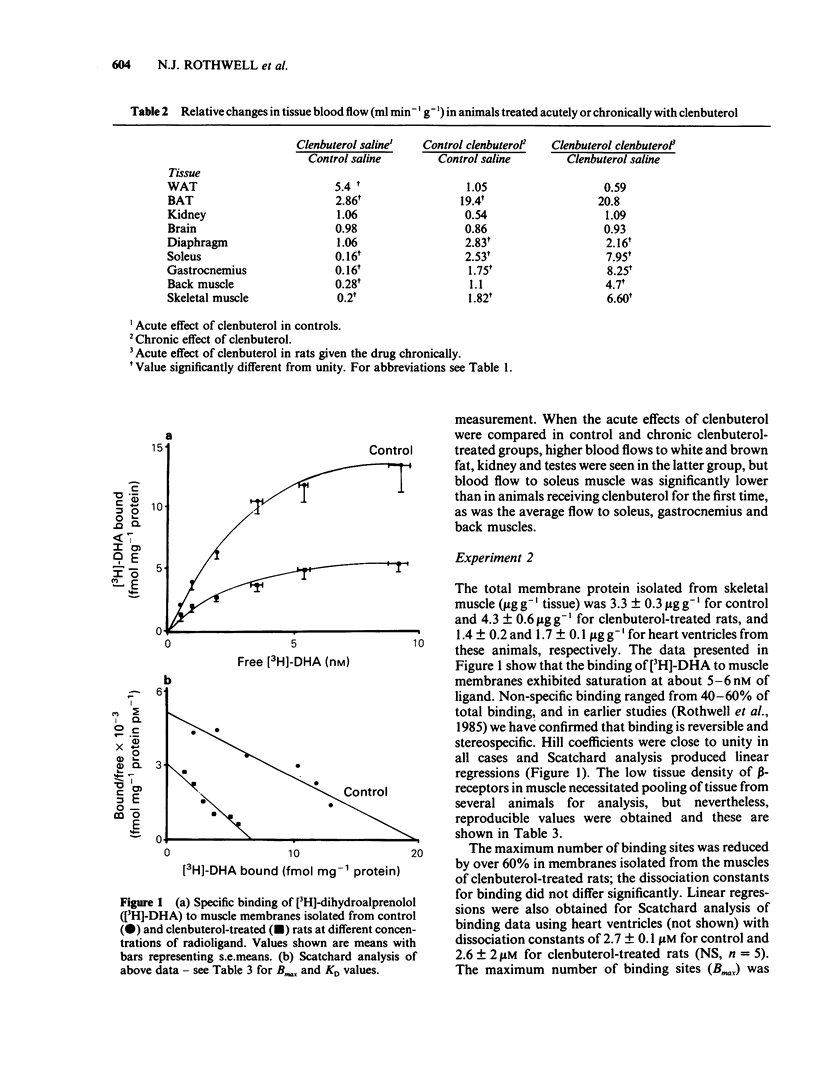
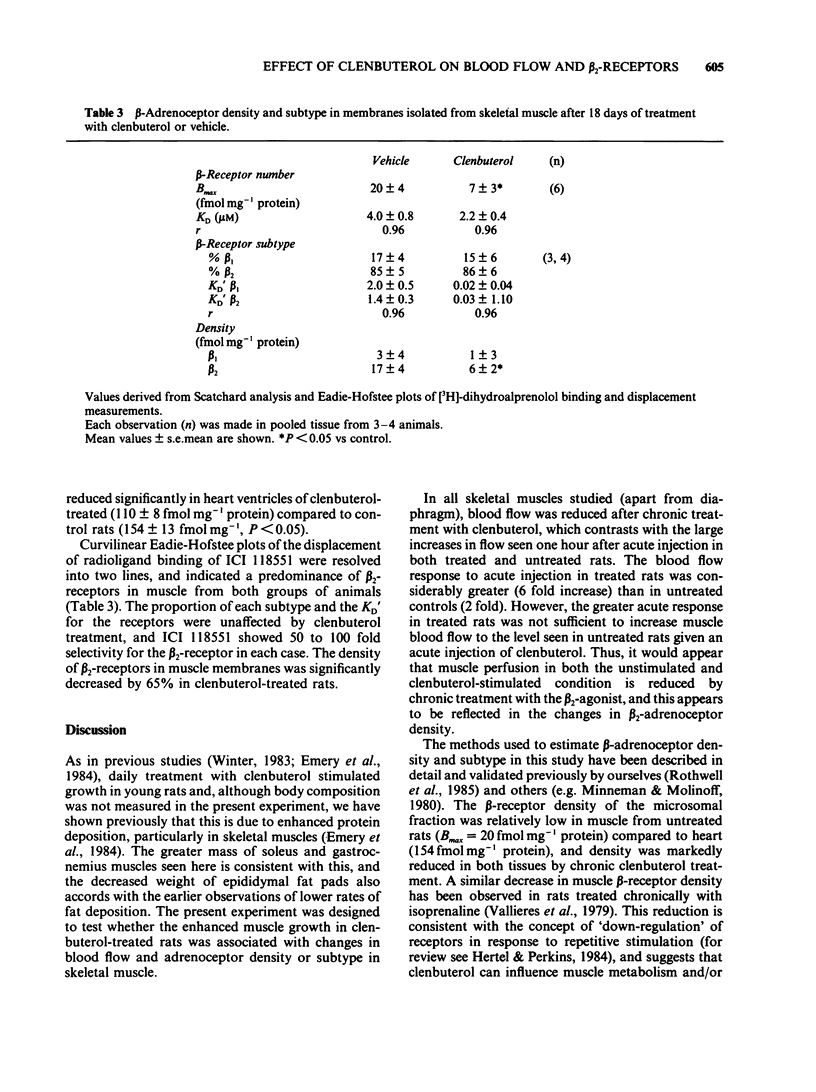
Rats injected with the beta2-adrenoceptor agonist clenbuterol (2 mg kg-1 per day) for 18 days gained significantly more weight than controls. Tissue blood flow assessed 24 h after the last injection from the distribution of radiolabelled microspheres was increased in white (5 fold) and brown (3 fold) adipose tissue of clenbuterol-treated rats but was unaffected in kidney, brain and diaphragm, and was reduced by about 80% in skeletal muscle. Acute injection of clenbuterol one hour before measuring blood flow, increased blood flow to brown fat (20 fold) in both treated and control groups. Blood flow to skeletal muscle increased more in the rats treated chronically with clenbuterol (6 fold increase) than in control rats (2 fold increase), but absolute flow rates were still significantly lower in the rats treated chronically with clenbuterol. Skeletal muscle beta-adrenoceptor density and subtype were assessed from ligand binding and displacement studies using [3H]-dihydroalprenolol. Rats treated with clenbuterol for 18 days showed a 50% reduction in beta-receptor density, but the ratio of beta 1/beta 2-receptors was unaffected (15% beta 1/85% beta 2). The results indicate that, although clenbuterol produces acute increases in muscle blood flow, chronic treatment results in lower flow rates immediately (1 h) and 24 h after the previous injection. The attenuated response following chronic treatment is associated with a marked reduction in skeletal muscle beta-adrenoceptor density. The data suggest that any anabolic effects of clenbuterol on muscle which may require, or may be mediated by increases in blood supply, cannot be sustained by chronic treatment.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emery P. W., Rothwell N. J., Stock M. J., Winter P. D. Chronic effects of beta 2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci Rep. 1984 Jan;4(1):83–91. doi: 10.1007/BF01120827. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Comparison of microspheres and 86Rb+ as tracers of the distribution of cardiac output in rats indicates invalidity of 86Rb+-based measurements. Can J Physiol Pharmacol. 1978 Feb;56(1):97–109. doi: 10.1139/y78-014. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol. 1978 Feb;56(1):110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- Hertel C., Perkins J. P. Receptor-specific mechanisms of desensitization of beta-adrenergic receptor function. Mol Cell Endocrinol. 1984 Oct;37(3):245–256. doi: 10.1016/0303-7207(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Molinoff P. B. Classification and quantitation of beta-adrenergic receptor subtypes. Biochem Pharmacol. 1980 May 15;29(10):1317–1323. doi: 10.1016/0006-2952(80)90424-4. [DOI] [PubMed] [Google Scholar]
- Reddy N. B., Engel W. K. In vitro characterization of skeletal muscle beta-adrenergic receptors coupled to adenylate cyclase. Biochim Biophys Acta. 1979 Jul 4;585(3):343–359. doi: 10.1016/0304-4165(79)90079-5. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Dorwood P. M., Palmer R. M. Stimulation of muscle growth by clenbuterol: lack of effect on muscle protein biosynthesis. Br J Nutr. 1986 Jul;56(1):249–258. doi: 10.1079/bjn19860104. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. Influence of noradrenaline on blood flow to brown adipose tissue in rats exhibiting diet-induced thermogenesis. Pflugers Arch. 1981 Mar;389(3):237–242. doi: 10.1007/BF00584784. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J., Stribling D. Diet-induced thermogenesis. Pharmacol Ther. 1982;17(2):251–268. doi: 10.1016/0163-7258(82)90016-x. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J., Sudera D. K. Beta-adrenoreceptors in rat brown adipose tissue: proportions of beta 1- and beta 2-subtypes. Am J Physiol. 1985 Apr;248(4 Pt 1):E397–E402. doi: 10.1152/ajpendo.1985.248.4.E397. [DOI] [PubMed] [Google Scholar]
- Vallières J., Cote C., Bukowiecki L. Regulation of beta-adrenergic receptors in rat skeletal muscle by catecholamines in vivo. Gen Pharmacol. 1979;10(1):63–67. doi: 10.1016/0306-3623(79)90031-4. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Caron M. G., Daniel K. Skeletal muscle beta-adrenergic receptors: variations due to fiber type and training. Am J Physiol. 1984 Feb;246(2 Pt 1):E160–E167. doi: 10.1152/ajpendo.1984.246.2.E160. [DOI] [PubMed] [Google Scholar]


