Abstract
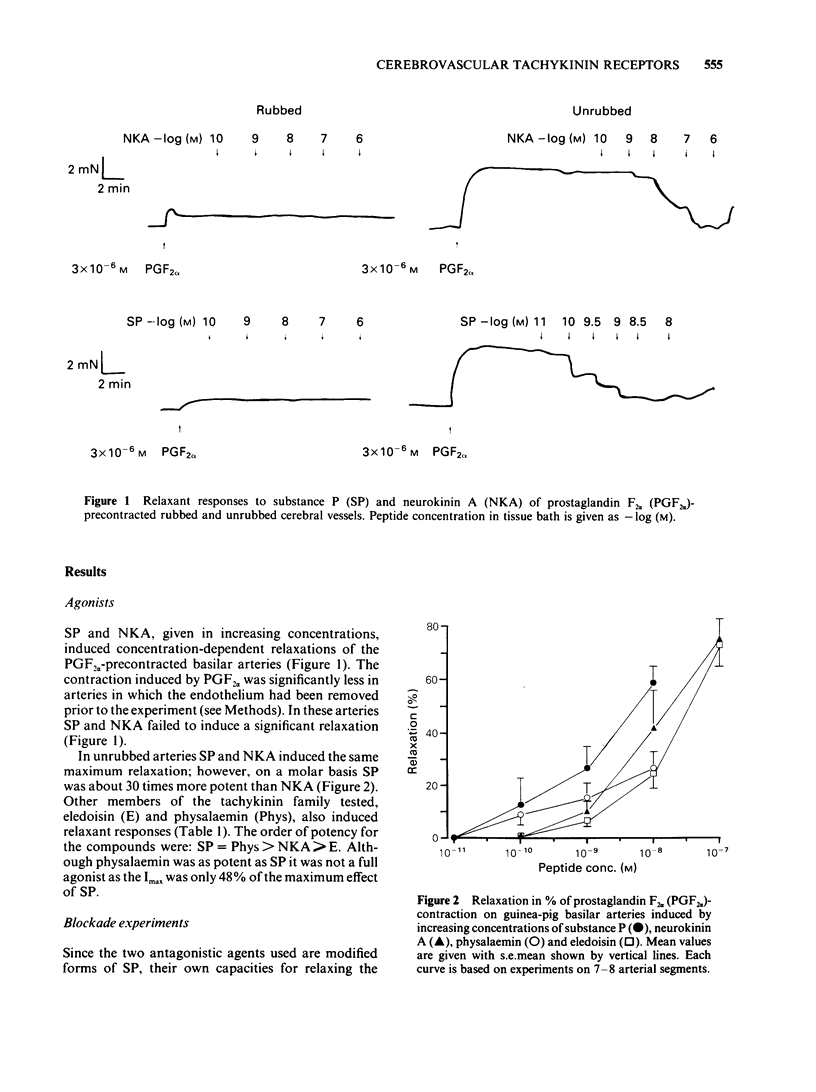
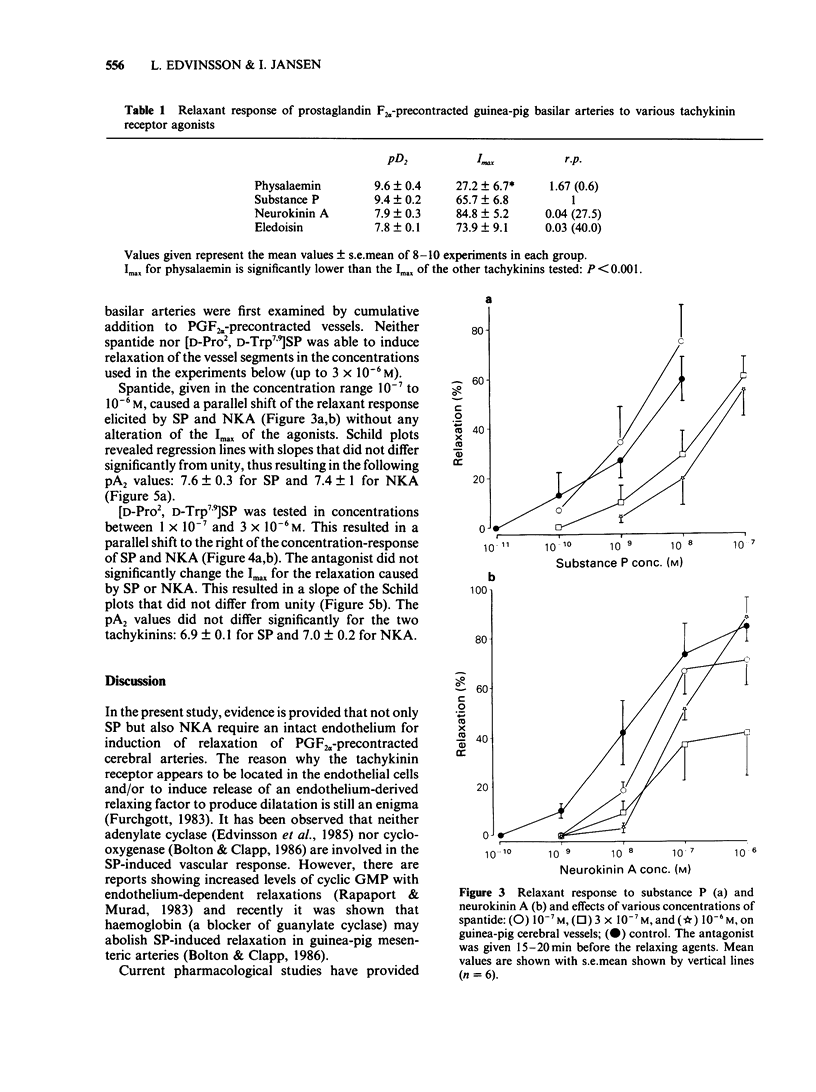
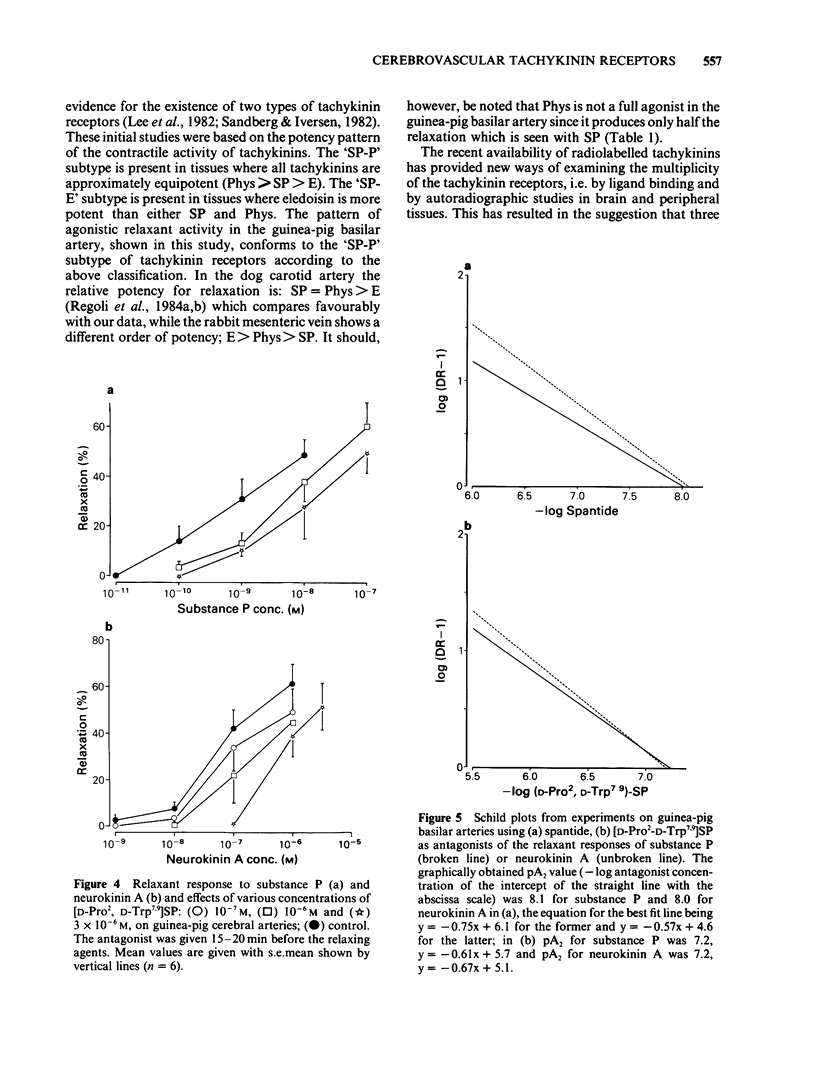
In circular lengths cut from the basilar artery of guinea-pig (0.2-0.3 mm o.d.) relaxations induced by substance P and neurokinin A were highly susceptible to mechanical damage of the endothelium by rubbing. The precontraction induced by prostaglandin F2 alpha but not that of 124 mM potassium was reduced considerably by the rubbing procedure. Concentration-dependent relaxations were evoked by tachykinin agonists in the following order of potency: substance P = physalaemin greater than neurokinin A greater than eledoisin. Physalaemin was, however, a partial agonist, giving only half the maximum relaxation as compared to the other tachykinins. The two putative tachykinin receptor antagonists, spantide ([D-Arg1, D-Trp7,9, D-Leu11] substance P) and [D-Pro2, D-Trp7,9] substance P, shifted the concentration-dependent relaxations of substance P to the right in a parallel manner. Calculation of pA2 values and Schild plot analysis revealed pA2 values of 7.4-7.6 for spantide and 6.9-7.0 for [D-Pro2, D-Trp7,9] substance P, irrespective of whether substance P or neurokinin A was used as agonist. The pA2 values and the Schild plot analysis suggest a specific interaction between tachykinin agonists and antagonists that follow a simple bimolecular process. The results suggest the presence of tachykinin receptors of the 'SP-P' type in guinea-pig basilar arteries which, for induction of relaxation, involves the release of an endothelium-derived relaxing factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaujouan J. C., Torrens Y., Viger A., Glowinski J. A new type of tachykinin binding site in the rat brain characterized by specific binding of a labeled eledoisin derivative. Mol Pharmacol. 1984 Sep;26(2):248–254. [PubMed] [Google Scholar]
- Bill A., Stjernschantz J., Mandahl A., Brodin E., Nilsson G. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol Scand. 1979 Jul;106(3):371–373. doi: 10.1111/j.1748-1716.1979.tb06412.x. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clapp L. H. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol. 1986 Apr;87(4):713–723. doi: 10.1111/j.1476-5381.1986.tb14589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S. H., Burcher E., Shults C. W., Lovenberg W., O'Donohue T. L. Novel pharmacology of substance K-binding sites: a third type of tachykinin receptor. Science. 1984 Nov 23;226(4677):987–989. doi: 10.1126/science.6095447. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Maurin Y., Burks T. F., Yamamura H. I. High-affinity 3H-substance P binding to longitudinal muscle membranes of the guinea pig small intestine. Life Sci. 1984 Jan 30;34(5):497–507. doi: 10.1016/0024-3205(84)90506-x. [DOI] [PubMed] [Google Scholar]
- Cascieri M. A., Chicchi G. G., Liang T. Demonstration of two distinct tachykinin receptors in rat brain cortex. J Biol Chem. 1985 Feb 10;260(3):1501–1507. [PubMed] [Google Scholar]
- Duckles S. P., Buck S. H. Substance P in the cerebral vasculature: depletion by capsaicin suggests a sensory role. Brain Res. 1982 Aug 5;245(1):171–174. doi: 10.1016/0006-8993(82)90355-9. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B., Hamel E., Jansen I., Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1985 Jul 31;58(2):213–217. doi: 10.1016/0304-3940(85)90166-1. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Feline cerebral veins and arteries: comparison of autonomic innervation and vasomotor responses. J Physiol. 1982 Apr;325:161–173. doi: 10.1113/jphysiol.1982.sp014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol. 1981 Sep;318:251–258. doi: 10.1113/jphysiol.1981.sp013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Gazelius B., Olgart L. Vasodilatation in the dental pulp produced by electrical stimulation of the inferior alveolar nerve in the cat. Acta Physiol Scand. 1980 Feb;108(2):181–186. doi: 10.1111/j.1748-1716.1980.tb06516.x. [DOI] [PubMed] [Google Scholar]
- Högestätt E. D., Andersson K. E., Edvinsson L. Mechanical properties of rat cerebral arteries as studied by a sensitive device for recording of mechanical activity in isolated small blood vessels. Acta Physiol Scand. 1983 Jan;117(1):49–61. doi: 10.1111/j.1748-1716.1983.tb07178.x. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. M., Iversen L. L., Hanley M. R., Sandberg B. E. The possible existence of multiple receptors for substance P. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):281–287. doi: 10.1007/BF00501166. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Javitch J. A., Snyder S. H. 3h-substance P binding to salivary gland membranes. Regulation by guanyl nucleotides and divalent cations. Mol Pharmacol. 1983 May;23(3):563–569. [PubMed] [Google Scholar]
- Lembeck F., Gamse R. Substance P in peripheral sensory processes. Ciba Found Symp. 1982;(91):35–54. doi: 10.1002/9780470720738.ch4. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Mayberg M. R., Moskowitz M. A. Immunohistochemical evidence for a substance P-containing trigeminovascular pathway to pial arteries in cats. Brain Res. 1983 May 23;268(1):162–166. doi: 10.1016/0006-8993(83)90402-x. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Brody M., Liu-Chen L. Y. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience. 1983 Aug;9(4):809–814. doi: 10.1016/0306-4522(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Park C. H., Massari V. J., Quirion R., Tizabi Y., Shults C. W., O'Donohue T. L. Characteristics of 3H-substance P binding sites in rat brain membranes. Peptides. 1984 Jul-Aug;5(4):833–836. doi: 10.1016/0196-9781(84)90031-7. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Regoli D., Escher E., Drapeau G., D'Orléans-Juste P., Mizrahi J. Receptors for substance P. III. Classification by competitive antagonists. Eur J Pharmacol. 1984 Jan 27;97(3-4):179–189. doi: 10.1016/0014-2999(84)90449-7. [DOI] [PubMed] [Google Scholar]
- Sandberg B. E., Iversen L. L. Substance P. J Med Chem. 1982 Sep;25(9):1009–1015. doi: 10.1021/jm00351a001. [DOI] [PubMed] [Google Scholar]
- Skärby T. V., Andersson K. E., Edvinsson L. Pharmacological characterization of postjunctional alpha-adrenoceptors in isolated feline cerebral and peripheral arteries. Acta Physiol Scand. 1983 Jan;117(1):63–73. doi: 10.1111/j.1748-1716.1983.tb07179.x. [DOI] [PubMed] [Google Scholar]
- Steiger H. J., Tew J. M., Jr, Keller J. T. The sensory representation of the dura mater in the trigeminal ganglion of the cat. Neurosci Lett. 1982 Aug 31;31(3):231–236. doi: 10.1016/0304-3940(82)90025-8. [DOI] [PubMed] [Google Scholar]
- Tallarida R. J., Cowan A., Adler M. W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979 Aug 20;25(8):637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- Tervo K., Tervo T., Eränkö L., Eränkö O., Cuello A. C. Immunoreactivity for substance P in the Gasserian ganglion, ophthalmic nerve and anterior segment of the rabbit eye. Histochem J. 1981 May;13(3):435–443. doi: 10.1007/BF01005059. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Ekman R., Kingman T., McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett. 1985 Nov 20;62(1):131–136. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Owman C., Sundler F. Perivascular substance P: occurrence and distribution in mammalian pial vessels. J Cereb Blood Flow Metab. 1981;1(2):227–232. doi: 10.1038/jcbfm.1981.24. [DOI] [PubMed] [Google Scholar]


