Abstract
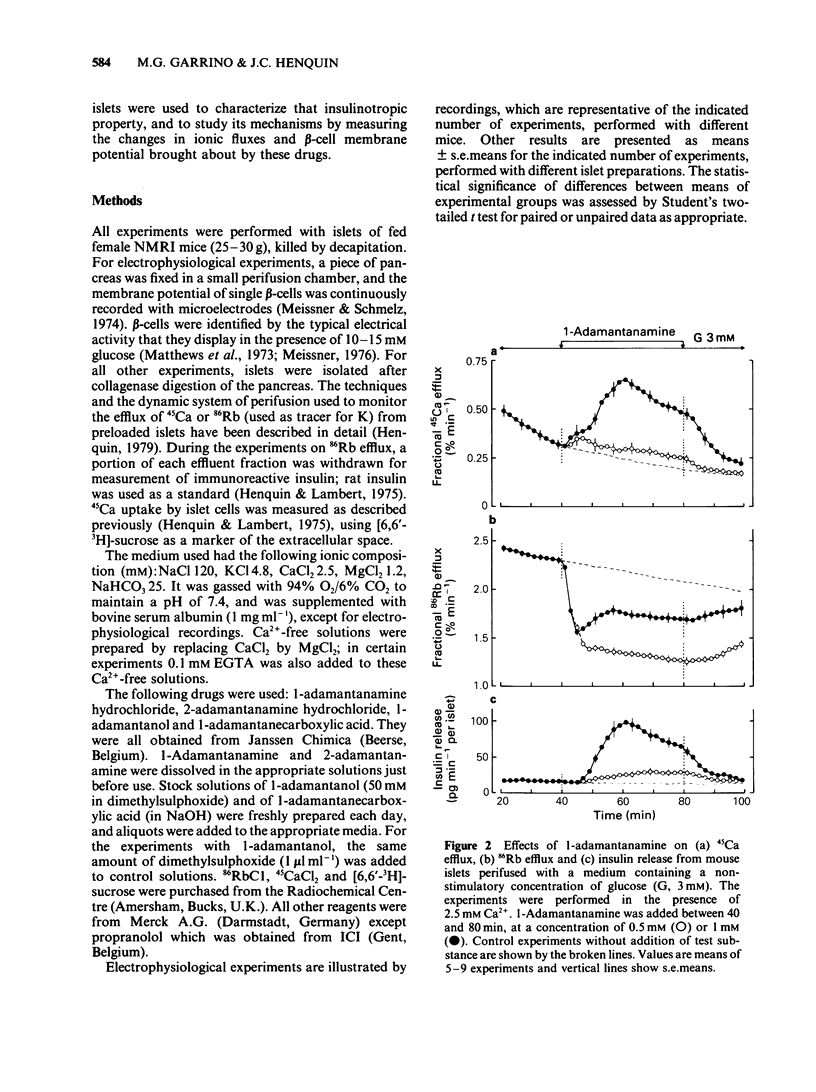
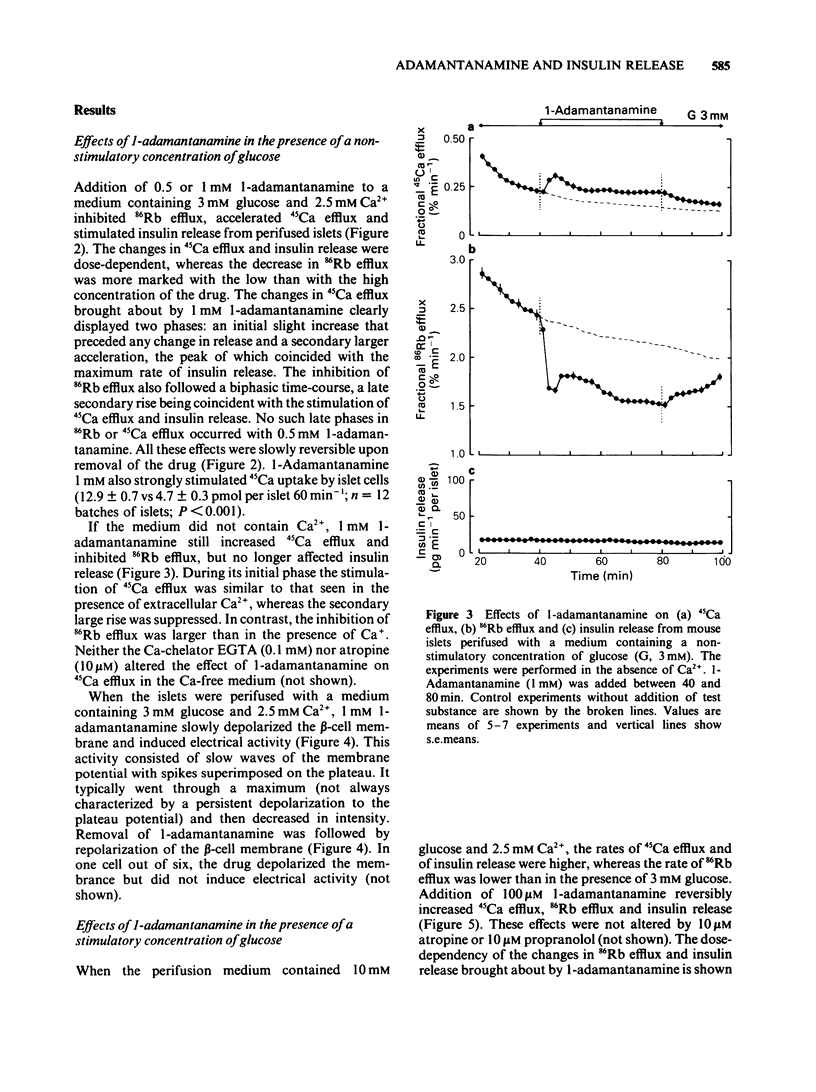
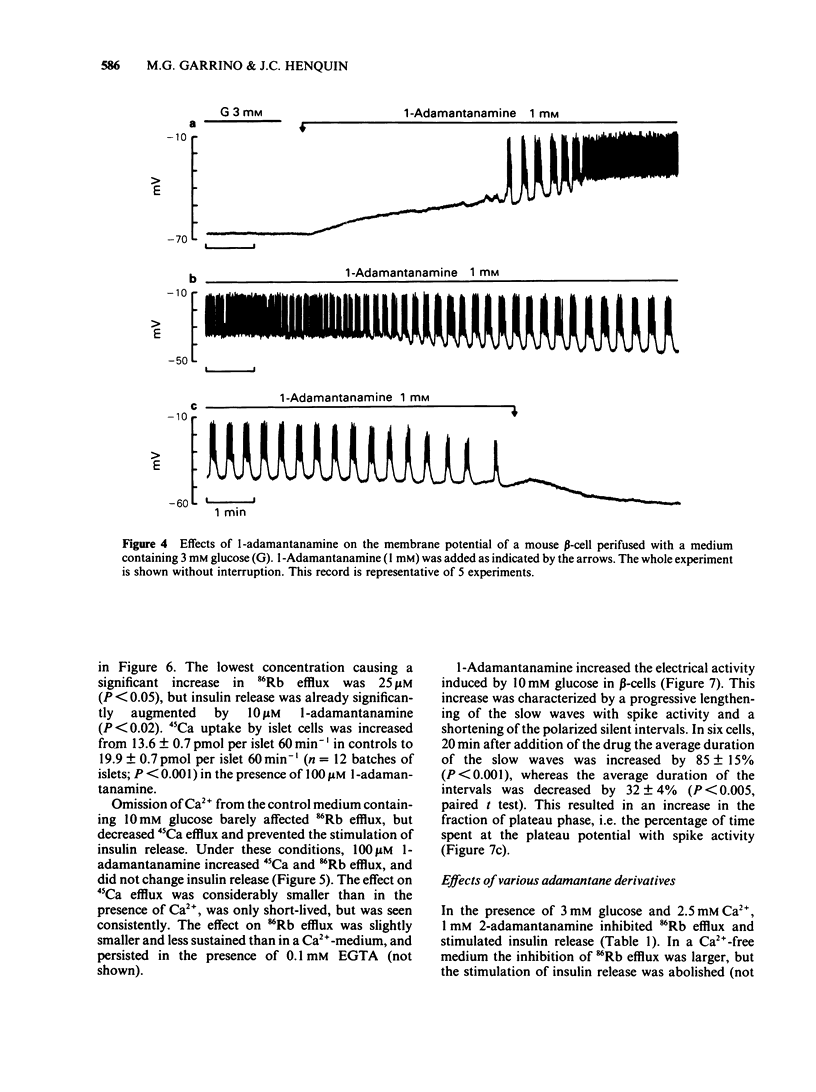
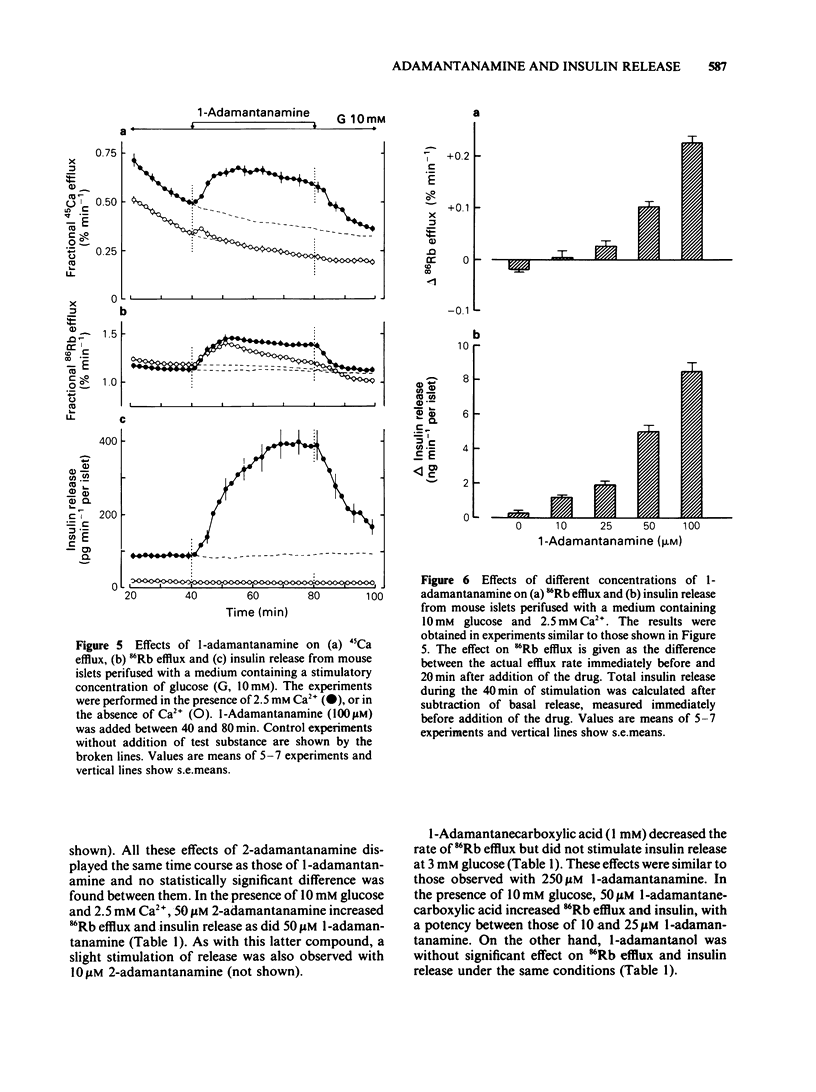
Adamantane derivatives were found to increase insulin release in vitro. Mouse islets were used to study the mechanisms and molecular requirements of that hitherto unrecognised property. At a non-stimulatory concentration of glucose (3 mM), 1-adamantanamine (1 mM) reversibly inhibited 86Rb efflux from islet cells, depolarized the beta-cell membrane, induced electrical activity, stimulated 45Ca uptake and efflux, and triggered insulin release. Omission of extracellular Ca2+ abolished the secretory response but only partially inhibited the acceleration of 45Ca efflux. At a stimulatory concentration of glucose (10 mM), 1-adamantanamine reversibly increased 86Rb efflux, potentiated electrical activity (lengthening of the slow waves with spikes), augmented 45Ca uptake and efflux, and increased insulin release. The effects of adamantanamine were dose-dependent, with a threshold concentration of 10 microM for stimulation release. 2-Adamantanamine was as potent as 1-adamantanamine. In contrast, substitution of the amino group by a carboxyl group (1-adamantanecarboxylic acid) decreased the effectiveness by about 65%, and substitution by a hydroxyl group (1-adamantanol) suppressed it. It is concluded that adamantane derivatives bearing an amino group decrease K+ permeability of the beta-cell membrane and thereby cause depolarization. This activates voltage-dependent Ca channels, permits Ca2+ influx and eventually stimulates insulin release. They may also mobilize cellular Ca2+, but this effect is not sufficient to cause release.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asplund K., Wiholm B. E., Lithner F. Glibenclamide-associated hypoglycaemia: a report on 57 cases. Diabetologia. 1983 Jun;24(6):412–417. doi: 10.1007/BF00257338. [DOI] [PubMed] [Google Scholar]
- Bailey E. V., Stone T. W. The mechanism of action of amantadine in Parkinsonism: a review. Arch Int Pharmacodyn Ther. 1975 Aug;216(2):246–262. [PubMed] [Google Scholar]
- Ferrer R., Atwater I., Omer E. M., Gonçalves A. A., Croghan P. C., Rojas E. Electrophysiological evidence for the inhibition of potassium permeability in pancreatic beta-cells by glibenclamide. Q J Exp Physiol. 1984 Oct;69(4):831–839. doi: 10.1113/expphysiol.1984.sp002872. [DOI] [PubMed] [Google Scholar]
- Freeman S. E., Dawson R. M., Culvenor A. J., Keeghan A. M. Interactions of amantadine with the cardiac muscarinic receptor. J Mol Cell Cardiol. 1985 Jan;17(1):9–21. doi: 10.1016/s0022-2828(85)80088-2. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Meissner H. P., Henquin J. C. The non-sulfonylurea moiety of gliquidone mimics the effects of the parent molecule on pancreatic B-cells. Eur J Pharmacol. 1986 May 27;124(3):309–316. doi: 10.1016/0014-2999(86)90232-3. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Schmeer W., Nenquin M., Meissner H. P., Henquin J. C. Mechanism of the stimulation of insulin release in vitro by HB 699, a benzoic acid derivative similar to the non-sulphonylurea moiety of glibenclamide. Diabetologia. 1985 Sep;28(9):697–703. doi: 10.1007/BF00291979. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Pelkonen R., Koskimies S., Bottazzo G. F., Doniach D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 1986 Mar-Apr;9(2):129–133. doi: 10.2337/diacare.9.2.129. [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Isaacson C. M., Boyajy L. D. Stimulation of insulin secretion from isolated rat islets by SaRI 59-801. Diabetes. 1985 Jun;34(6):548–552. doi: 10.2337/diab.34.6.548. [DOI] [PubMed] [Google Scholar]
- Hellman B. Tolbutamide stimulation of 45Ca fluxes in microdissected pancreatic islets rich in beta-cells. Mol Pharmacol. 1981 Jul;20(1):83–88. [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Cobalt inhibition of insulin secretion and calcium uptake by isolated rat islets. Am J Physiol. 1975 Jun;228(6):1669–1677. doi: 10.1152/ajplegacy.1975.228.6.1669. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Tolbutamide stimulation and inhibition of insulin release: studies of the underlying ionic mechanisms in isolated rat islets. Diabetologia. 1980;18(2):151–160. doi: 10.1007/BF00290493. [DOI] [PubMed] [Google Scholar]
- Iwai H., Inamasu M., Totsuka T., Shimazaki T., Morita T., Takeyama S. Hypoglycemic activity of 1-alpha-(3,4-dimethoxyphenethylaminomethyl) -2-hydroxybenzylalcohol 1/2 fumarate (TA-078) in the mouse, rat and dog. Biochem Pharmacol. 1983 Mar 1;32(5):849–855. doi: 10.1016/0006-2952(83)90587-7. [DOI] [PubMed] [Google Scholar]
- Kameda K. Y., Koyama I., Abiko Y. Comparative study on the effects of a hypoglycemic 2-substituted-2-imidazoline derivative (DG-5128) and tolbutamide on insulin secretion from and insulin synthesis in the isolated rat pancreatic islets. Biochim Biophys Acta. 1981 Oct 12;677(2):263–268. doi: 10.1016/0304-4165(81)90094-5. [DOI] [PubMed] [Google Scholar]
- Lebrun P., Malaisse W. J., Herchuelz A. Modalities of gliclazide-induced Ca2+ influx into the pancreatic B-cell. Diabetes. 1982 Nov;31(11):1010–1015. doi: 10.2337/diacare.31.11.1010. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Shotton P. A. Efflux of 86Rb from rat and mouse pancreatic islets: the role of membrane depolarization. Br J Pharmacol. 1984 Nov;83(3):831–839. doi: 10.1111/j.1476-5381.1984.tb16239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Shotton P. A. The control of 86Rb efflux from rat isolated pancreatic islets by the sulphonylureas tolbutamide and glibenclamide. Br J Pharmacol. 1984 Jul;82(3):689–700. doi: 10.1111/j.1476-5381.1984.tb10808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H. P. Electrical characteristics of the beta-cells in pancreatic islets. J Physiol (Paris) 1976 Nov;72(6):757–767. [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Mészáros J., Kelemen K., Markó R., Kecskeméti V., Szegi J. Inhibition of myocardial K+ channels by bromobenzoyl-methyladamantylamine, an adamantane derivative. Eur J Pharmacol. 1982 Oct 22;84(3-4):151–160. doi: 10.1016/0014-2999(82)90197-2. [DOI] [PubMed] [Google Scholar]
- Nenquin M., Awouters P., Mathot F., Henquin J. C. Distinct effects of acetylcholine and glucose on 45calcium and 86rubidium efflux from mouse pancreatic islets. FEBS Lett. 1984 Oct 29;176(2):457–461. doi: 10.1016/0014-5793(84)81218-1. [DOI] [PubMed] [Google Scholar]
- Parkes D. Amantadine. Adv Drug Res. 1974;8:11–81. [PubMed] [Google Scholar]
- Quickel K. E., Jr, Feldman J. M., Lebovitz H. E. Inhibition of insulin secretion by serotonin and dopamine: species variation. Endocrinology. 1971 Nov;89(5):1295–1302. doi: 10.1210/endo-89-5-1295. [DOI] [PubMed] [Google Scholar]
- Schnur R. C., Morville M. Improved glucose tolerance in rats treated with oxazolidinediones. J Med Chem. 1986 May;29(5):770–778. doi: 10.1021/jm00155a030. [DOI] [PubMed] [Google Scholar]
- Vernier V. G., Harmon J. B., Stump J. M., Lynes T. E., Marvel J. P., Smith D. H. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol Appl Pharmacol. 1969 Nov;15(3):642–665. doi: 10.1016/0041-008x(69)90066-0. [DOI] [PubMed] [Google Scholar]
- Warnick J. E., Maleque M. A., Bakry N., Eldefrawi A. T., Albuquerque E. X. Structure-activity relationships of amantadine. I. Interaction of the N-alkyl analogues with the ionic channels of the nicotinic acetylcholine receptor and electrically excitable membrane. Mol Pharmacol. 1982 Jul;22(1):82–93. [PubMed] [Google Scholar]
- van Ackern K., Deuster J. E., Mast G. J., Schmier J. Wirkungen von Amantadin auf Herz und Kreislauf. Arzneimittelforschung. 1975 Jun;25(6):891–896. [PubMed] [Google Scholar]


