Abstract
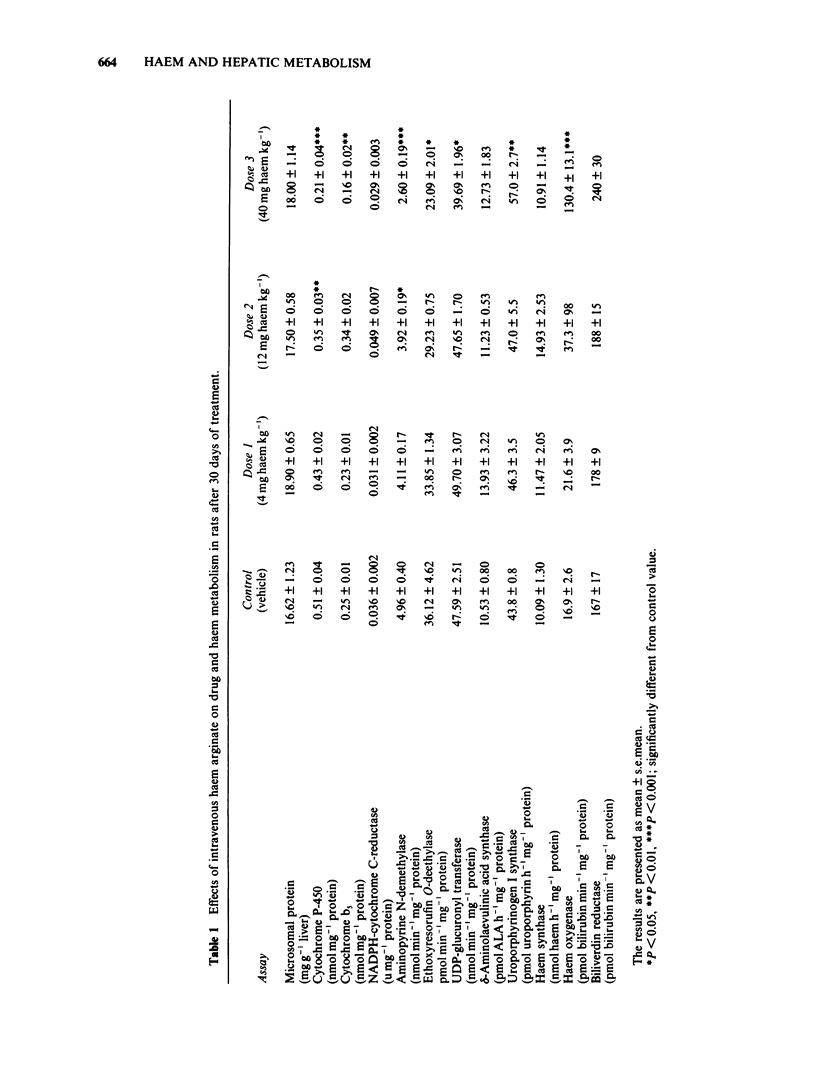
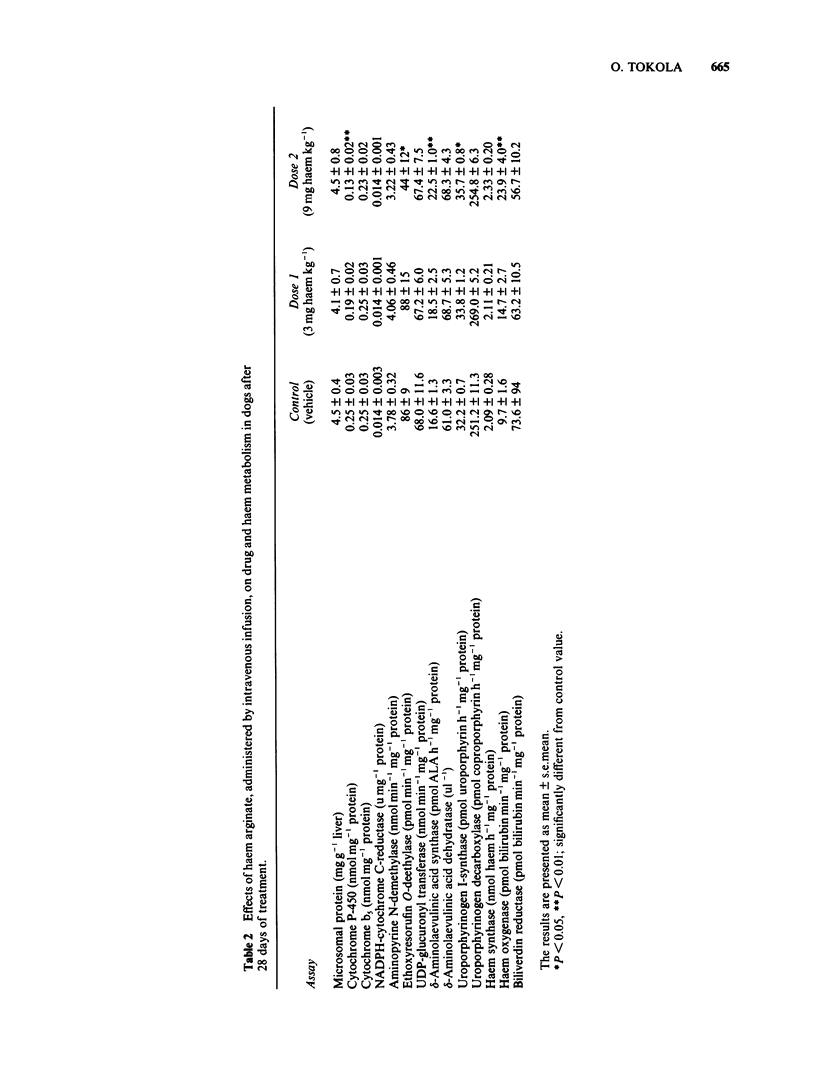
Haem arginate is a new haem compound, recently introduced for the treatment of acute hepatic porphyrias. Porphyrias are characterized biochemically by decreased formation of haem due to defects in certain enzyme activities involved in the haem biosynthesis. Haem is essential for cell respiration and oxidative biotransformation. Hepatic drug metabolism, haem biosynthesis and catabolism were investigated after repeated intravenous administration of haem arginate in connection with toxicity studies. The daily doses of haem for rats were 4, 12 and 40 mg kg-1 and for dogs 3 and 9 mg kg-1 for 30 days and for 28 days, respectively. Hepatic microsomes were used in the assay of the following drug metabolizing enzymes: cytochrome P-450 and b5, aminopyrine N-demethylase, ethoxyresorufin O-deethylase and UDP-glucuronyl transferase. The assay of NADPH-cytochrome C-reductase and the enzymes reflecting synthesis and metabolism of haem in the liver (delta-aminolaevulinic acid synthase, delta-aminolaevulinic acid dehydratase, uroporphyrinogen I-synthase, uroporphyrinogen decarboxylase, haem synthase, haem oxygenase and biliverdin reductase) were performed from 20,000 g supernatants. The lowest dose administered to rats and dogs did not cause any significant changes compared to controls in the parameters measured. The highest doses significantly increased the activities of haem oxygenase and uroporphyrinogen I-synthase but decreased concentrations or activities of other enzymes, e.g. cytochrome P-450 and ethoxyresorufin O-deethylase. The results show that it is important to avoid overdosage of haem when restoration of mixed function oxygenase activity is needed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Alvares A. P., Sassa S., Kappas A. Studies in porphyria. V. Drug oxidation rates in hereditary hepatic porphyria. Clin Pharmacol Ther. 1976 Jan;19(1):47–54. doi: 10.1002/cpt197619147. [DOI] [PubMed] [Google Scholar]
- Bauer J., Fornnarino J. High-performance liquid chromatographic analysis of "available" hemin in hematin solutions. J Chromatogr. 1984 Jan 20;283:378–382. doi: 10.1016/s0021-9673(00)96276-9. [DOI] [PubMed] [Google Scholar]
- Berlin A., Schaller K. H. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem. 1974 Aug;12(8):389–390. [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M. D., Mayer R. T. Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos. 1974 Nov-Dec;2(6):583–588. [PubMed] [Google Scholar]
- Cinti D. L., Moldeus P., Schenkman J. B. Kinetic parameters of drug-metabolizing enzymes in Ca 2+ -sedimented microsomes from rat liver. Biochem Pharmacol. 1972 Dec 15;21(24):3249–3256. doi: 10.1016/0006-2952(72)90089-5. [DOI] [PubMed] [Google Scholar]
- Ebert P. S., Tschudy D. P., Choudhry J. N., Chirigos M. A. A simple micro method for the direct determination of delta-amino (14C) levulinic acid production in murine spleen and liver homogenates. Biochim Biophys Acta. 1970 May 12;208(2):236–250. doi: 10.1016/0304-4165(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Farrell G. C., Correia M. A. Structural and functional reconstitution of hepatic cytochrome P-450 in vivo. Reversal of allylisopropylacetamide-mediated destruction of the hemoprotein by exogenous heme. J Biol Chem. 1980 Nov 10;255(21):10128–10133. [PubMed] [Google Scholar]
- Farrell G. C., Zaluzny L. Hepatic heme metabolism and cytochrome P450 in cirrhotic rat liver. Gastroenterology. 1985 Jul;89(1):172–179. doi: 10.1016/0016-5085(85)90759-0. [DOI] [PubMed] [Google Scholar]
- Goetsch C. A., Bissell D. M. Instability of hematin used in the treatment of acute hepatic porphyria. N Engl J Med. 1986 Jul 24;315(4):235–238. doi: 10.1056/NEJM198607243150406. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kitchin K. T. Laboratory methods for ten hepatic toxification/detoxification parameters. Methods Find Exp Clin Pharmacol. 1983 Sep;5(7):439–448. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. The induction of heme oxidation in various tissues by trace metals: evidence for the catabolism of endogenous heme by hepatic heme oxygenase. Ann Clin Res. 1976;8 (Suppl 17):39–46. [PubMed] [Google Scholar]
- McKillop D. Effects of phenobarbitone and beta-naphthoflavone on hepatic microsomal drug metabolising enzymes of the male beagle dog. Biochem Pharmacol. 1985 Sep 1;34(17):3137–3142. doi: 10.1016/0006-2952(85)90159-5. [DOI] [PubMed] [Google Scholar]
- Mendenhall D. W. Instability of hematin solutions. N Engl J Med. 1984 Aug 23;311(8):539–539. doi: 10.1056/NEJM198408233110819. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard U., Eiseman J. L., Foidart M., Alvares A. P. Effect of heme on allylisopropylacetamide-induced changes in heme and drug metabolism in the rhesus monkey (Macaca mulatta). Biochem Pharmacol. 1983 Dec 15;32(24):3765–3769. doi: 10.1016/0006-2952(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Mustajoki P. Red cell uroporphyrinogen I synthetase in acute intermittent porphyria. Ann Clin Res. 1976;8 (Suppl 17):133–138. [PubMed] [Google Scholar]
- Namkung M. J., Faustman-Watts E., Juchau M. R. Hematin-mediated increases of benzo(a)pyrene mono-oxygenation in maternal, fetal and placental tissues of inducible and non-inducible mouse strains. Dev Pharmacol Ther. 1983;6(3):199–206. doi: 10.1159/000457295. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Oritz de Montellano P. R., Correia M. A. Suicidal destruction of cytochrome P-450 during oxidative drug metabolism. Annu Rev Pharmacol Toxicol. 1983;23:481–503. doi: 10.1146/annurev.pa.23.040183.002405. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Kaltiala E. H., Larmi T. K., Kärki N. T. Comparison of activities of drug-metabolizing enzymes in human fetal and adult livers. Clin Pharmacol Ther. 1973 Sep-Oct;14(5):840–846. doi: 10.1002/cpt1973145840. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Pasanen M., Kuha H., Gachalyi B., Kairaluoma M., Sotaniemi E. A., Park S. S., Friedman F. K., Gelboin H. V. The effect of cigarette smoking on 7-ethoxyresorufin O-deethylase and other monooxygenase activities in human liver: analyses with monoclonal antibodies. Br J Clin Pharmacol. 1986 Aug;22(2):125–134. doi: 10.1111/j.1365-2125.1986.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierach C. A. Hematin therapy for the porphyric attack. Semin Liver Dis. 1982 May;2(2):125–131. doi: 10.1055/s-2008-1040702. [DOI] [PubMed] [Google Scholar]
- Romeo G., Levin E. Y. Uroporphyrinogen decarboxylase from mouse spleen. Biochim Biophys Acta. 1971 Feb 23;230(2):330–341. doi: 10.1016/0304-4165(71)90220-0. [DOI] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Ross M. E., Marver H. S., Schmid R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970 Jan 20;9(2):298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Savolainen H., Jäppinen P. Changes in haem synthesis associated with occupational exposure to organic and inorganic sulphides. Clin Sci (Lond) 1983 Feb;64(2):187–191. doi: 10.1042/cs0640187. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Zitting A., Nickels J., Savolainen H. Trinitrotoluene-induced effects on rat heme metabolism. Exp Mol Pathol. 1984 Jun;40(3):362–366. doi: 10.1016/0014-4800(84)90053-4. [DOI] [PubMed] [Google Scholar]
- Watson C. J., Dhar G. J., Bossenmaier I., Cardinal R., Petryka Z. J. Effect of hematin in acute porphyric relapse. Ann Intern Med. 1973 Jul;79(1):80–83. doi: 10.7326/0003-4819-79-1-80. [DOI] [PubMed] [Google Scholar]
- von Bahr C., Groth C. G., Jansson H., Lundgren G., Lind M., Glaumann H. Drug metabolism in human liver in vitro: establishment of a human liver bank. Clin Pharmacol Ther. 1980 Jun;27(6):711–725. doi: 10.1038/clpt.1980.102. [DOI] [PubMed] [Google Scholar]


