Abstract
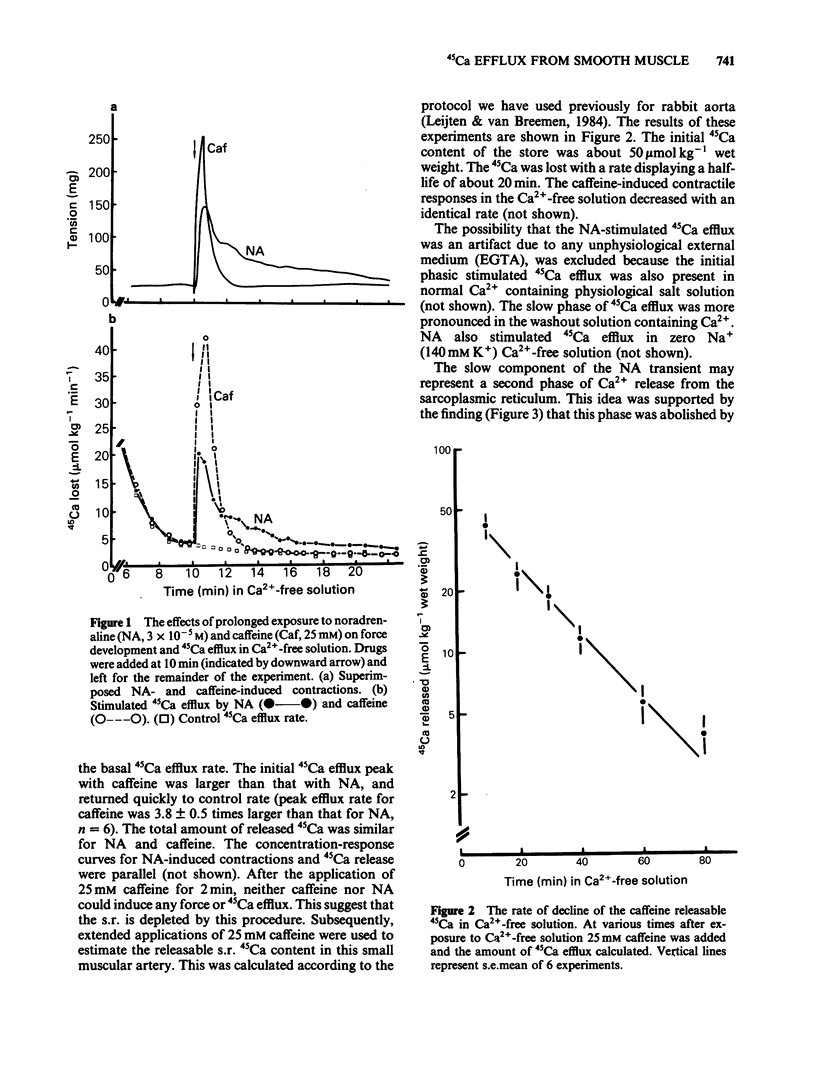
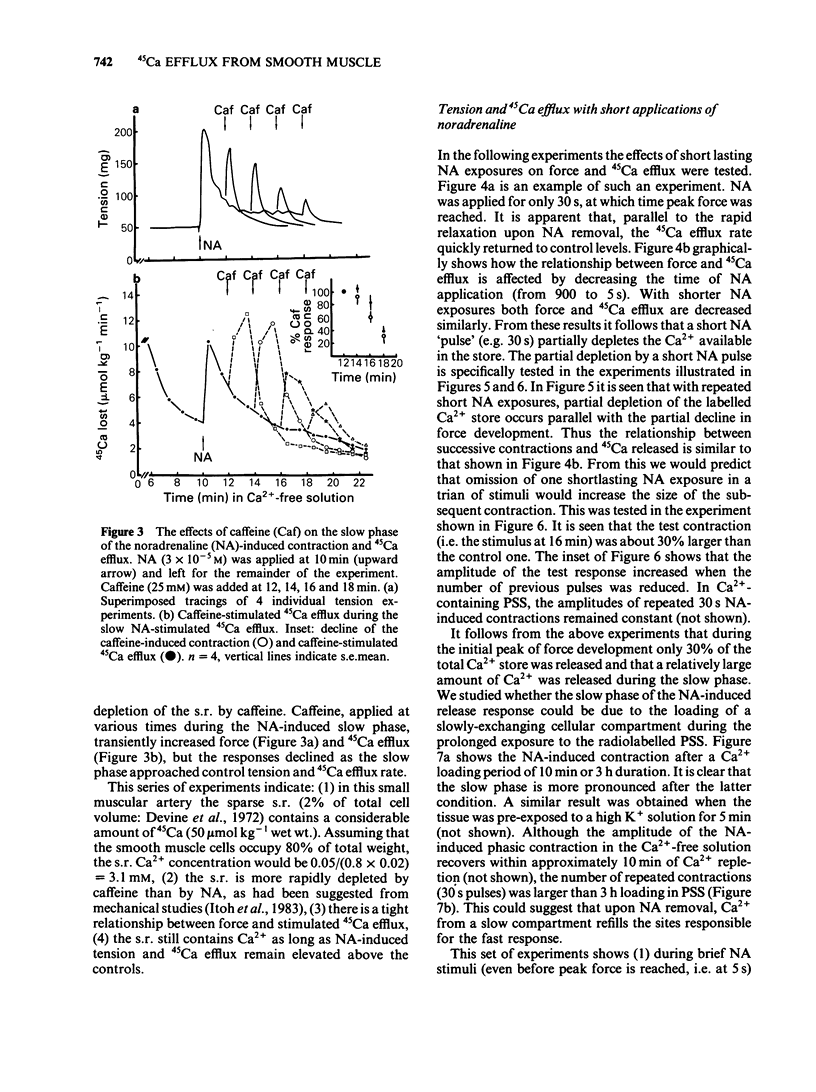
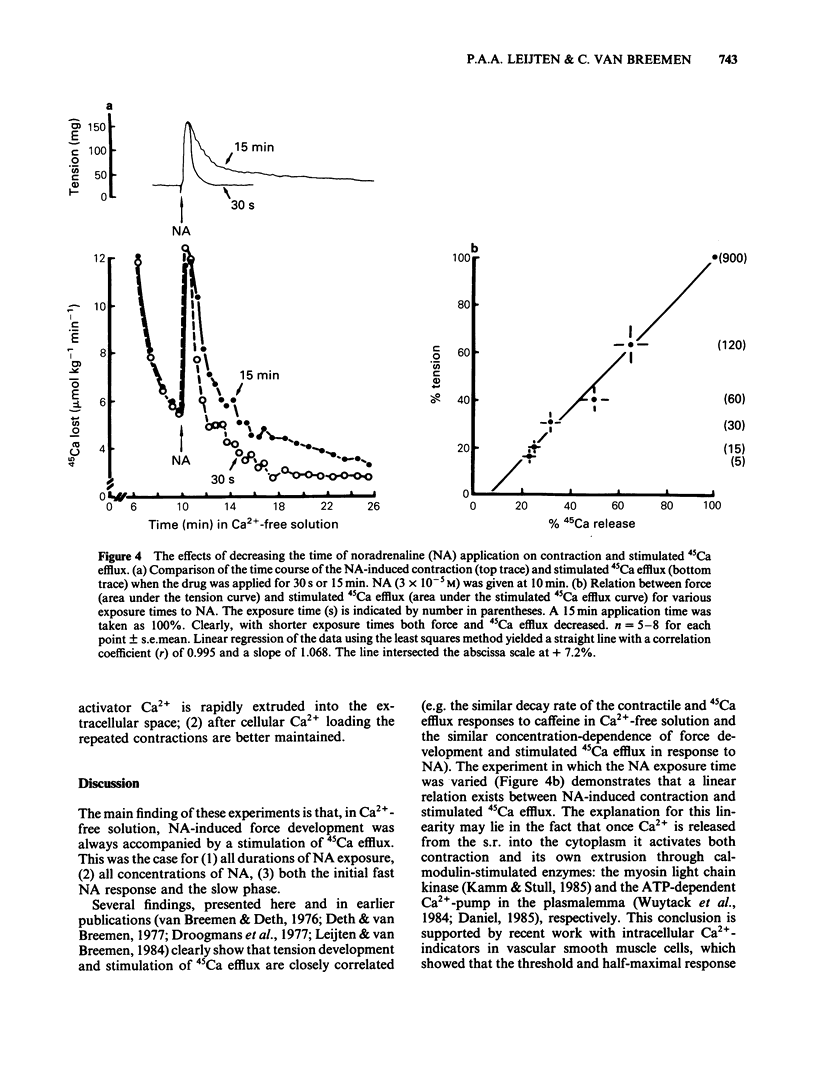
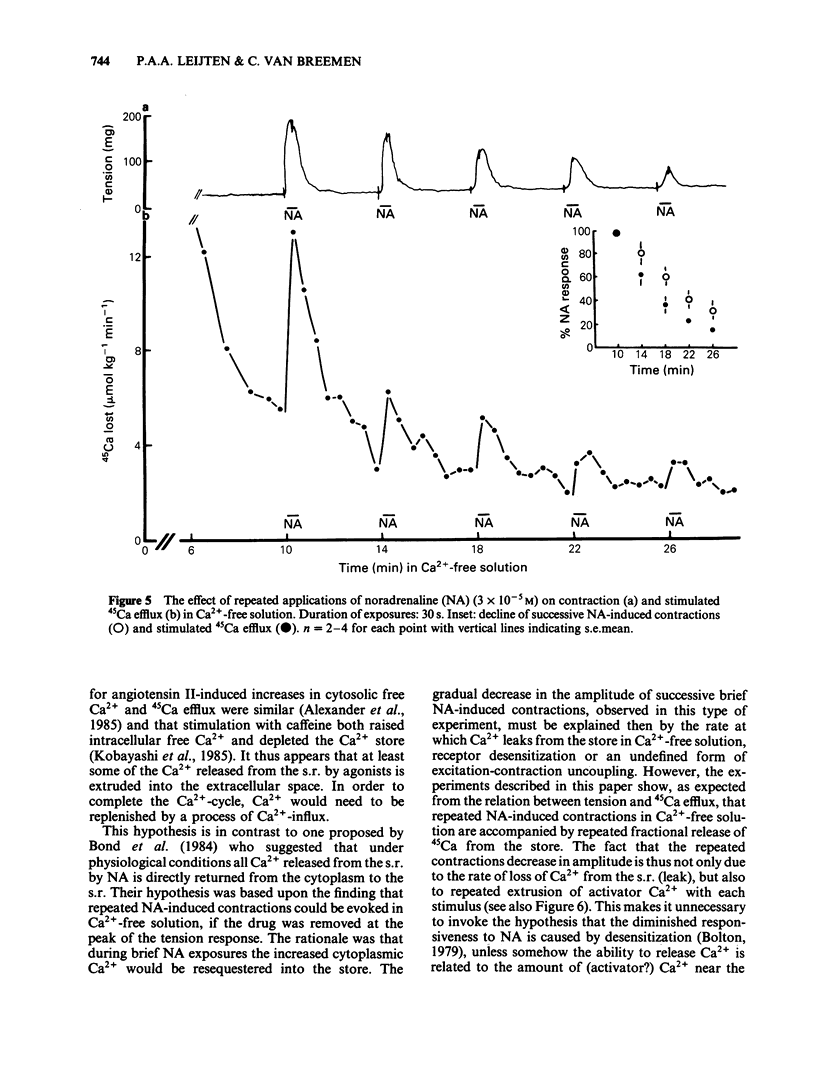
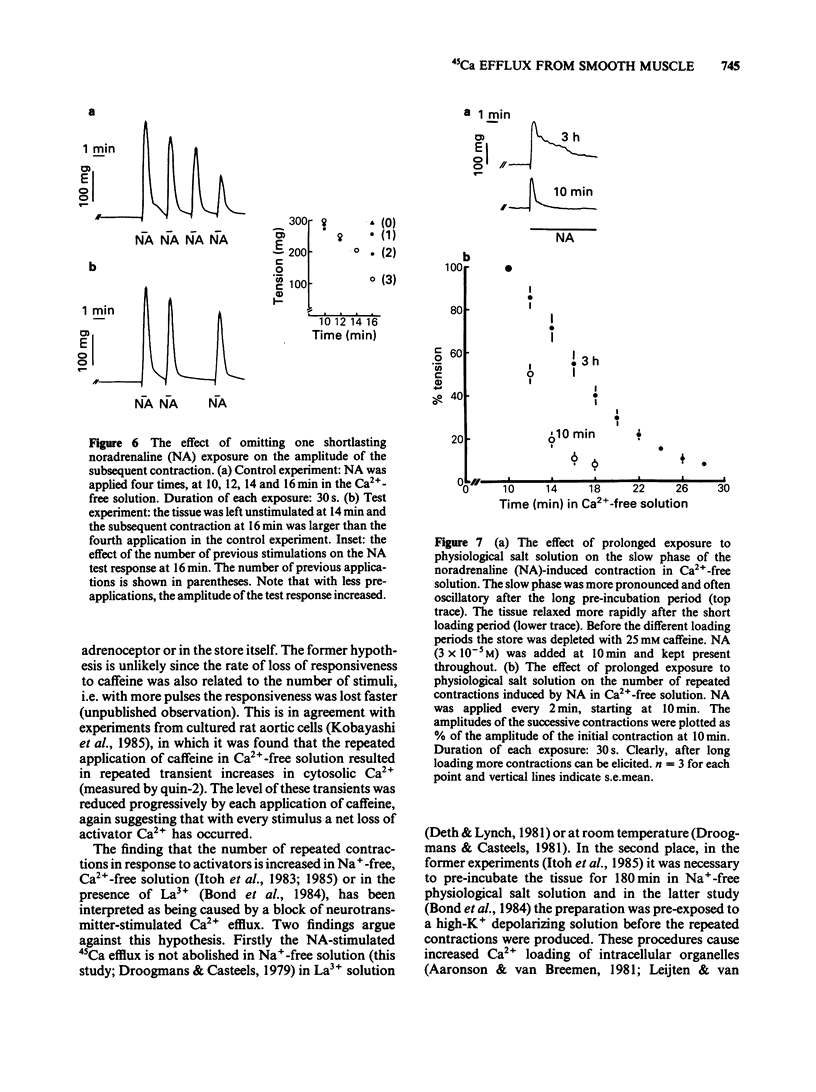
Cellular Ca2+ recycling in a branch of the rabbit mesenteric artery was investigated by measuring the time- and concentration-dependent effects of noradrenaline (NA) on contraction and 45Ca efflux in Ca2+-free solution. When NA was present continuously (15 min), both force development and 45Ca efflux stimulation consisted of a fast and a slow (often oscillatory) component. These components were sensitive to caffeine and are probably both related to Ca2+ release from the intracellular Ca2+ store, presumably sarcoplasmic reticulum (s.r.). When NA was applied for shorter time periods, both tension and stimulated 45Ca efflux decreased similarly. Repetitive short (30 s) NA applications resulted in repeated contractions and stimulations of 45Ca efflux. The NA-stimulated 45Ca efflux was not inhibited when external Ca2+ was present or in Na+-free medium. Loading the cell with Ca2+ (with physiological salt solution for 3 h or with a high K+ depolarizing solution) increases the number of subsequent NA-induced repeated contractions in Ca2+-free solution. The Ca2+ content of the sarcoplasmic reticulum (s.r.) in the smooth muscle cells of this small artery was estimated to be at least 50 mumol kg-1 wet weight, corresponding to an s.r. Ca2+ concentration of about 3.1 mM. These results indicate that the NA-induced increase in cytosolic free Ca2+ (as measured by force development) is accompanied by an increase in Ca2+ extrusion (as measured by stimulation of 45Ca efflux). This suggests that at least part of the activator Ca2+ cycles through the extracellular space during hormone-induced activation of vascular smooth muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P., van Breemen C. Effects of sodium gradient manipulation upon cellular calcium, 45Ca fluxes and cellular sodium in the guinea-pig taenia coli. J Physiol. 1981;319:443–461. doi: 10.1113/jphysiol.1981.sp013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R. W., Brock T. A., Gimbrone M. A., Jr, Rittenhouse S. E. Angiotensin increases inositol trisphosphate and calcium in vascular smooth muscle. Hypertension. 1985 May-Jun;7(3 Pt 1):447–451. [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E. The use of subcellular membrane fractions in analysis of control of smooth muscle function. Experientia. 1985 Jul 15;41(7):905–913. doi: 10.1007/BF01970009. [DOI] [PubMed] [Google Scholar]
- Deth R. C., Lynch C. J. Mobilization of a common source of smooth muscle Ca2+ by norepinephrine and methylxanthines. Am J Physiol. 1981 May;240(5):C239–C247. doi: 10.1152/ajpcell.1981.240.5.C239. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Casteels R. Sodium and calcium interactions in vascular smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1979 Jul;74(1):57–70. doi: 10.1085/jgp.74.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Casteels R. Temperature-dependence of 45Ca fluxes and contraction in vascular smooth muscle cells of rabbit ear artery. Pflugers Arch. 1981 Sep;391(3):183–189. doi: 10.1007/BF00596168. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Rapid ionic modifications during the aequorin-detected calcium transient in a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):189–246. doi: 10.1085/jgp.85.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Wier W. G. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of caffeine on the intracellular [Ca2+] transient, membrane currents, and contraction. J Gen Physiol. 1984 Mar;83(3):417–433. doi: 10.1085/jgp.83.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Suzuki H., Kuriyama H. On the roles of calcium ion during potassium induced contracture in the smooth muscle cells of the rabbit main pulmonary artery. Jpn J Physiol. 1977;27(6):755–770. doi: 10.2170/jjphysiol.27.755. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Sasaguri T., Makita Y., Kanmura Y., Kuriyama H. Mechanisms of vasodilation induced by vasoactive intestinal polypeptide in rabbit mesenteric artery. Am J Physiol. 1985 Aug;249(2 Pt 2):H231–H240. doi: 10.1152/ajpheart.1985.249.2.H231. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Karaki H., Kubota H., Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979 Jun 15;56(3):237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kanaide H., Nakamura M. Cytosolic-free calcium transients in cultured vascular smooth muscle cells: microfluorometric measurements. Science. 1985 Aug 9;229(4713):553–556. doi: 10.1126/science.3927484. [DOI] [PubMed] [Google Scholar]
- Kowarski D., Shuman H., Somlyo A. P., Somlyo A. V. Calcium release by noradrenaline from central sarcoplasmic reticulum in rabbit main pulmonary artery smooth muscle. J Physiol. 1985 Sep;366:153–175. doi: 10.1113/jphysiol.1985.sp015790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijten P., Saida K., van Breemen C. Norepinephrine-induced intracellular Ca2+ release from vascular smooth muscle. J Cardiovasc Pharmacol. 1985;7 (Suppl 6):S38–S42. doi: 10.1097/00005344-198500076-00007. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P. Excitation-contraction coupling and the ultrastructure of smooth muscle. Circ Res. 1985 Oct;57(4):497–507. doi: 10.1161/01.res.57.4.497. [DOI] [PubMed] [Google Scholar]
- Vonderlage M. Changes in the fast component of the rabbit vena cava contraction after repeated stimulation by noradrenaline(NA). Eur J Pharmacol. 1976 Mar;36(1):61–67. doi: 10.1016/0014-2999(76)90257-0. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L., Verbist J., De Smedt H., Casteels R. Evidence for the presence in smooth muscle of two types of Ca2+-transport ATPase. Biochem J. 1984 Dec 1;224(2):445–451. doi: 10.1042/bj2240445. [DOI] [PMC free article] [PubMed] [Google Scholar]


