Abstract
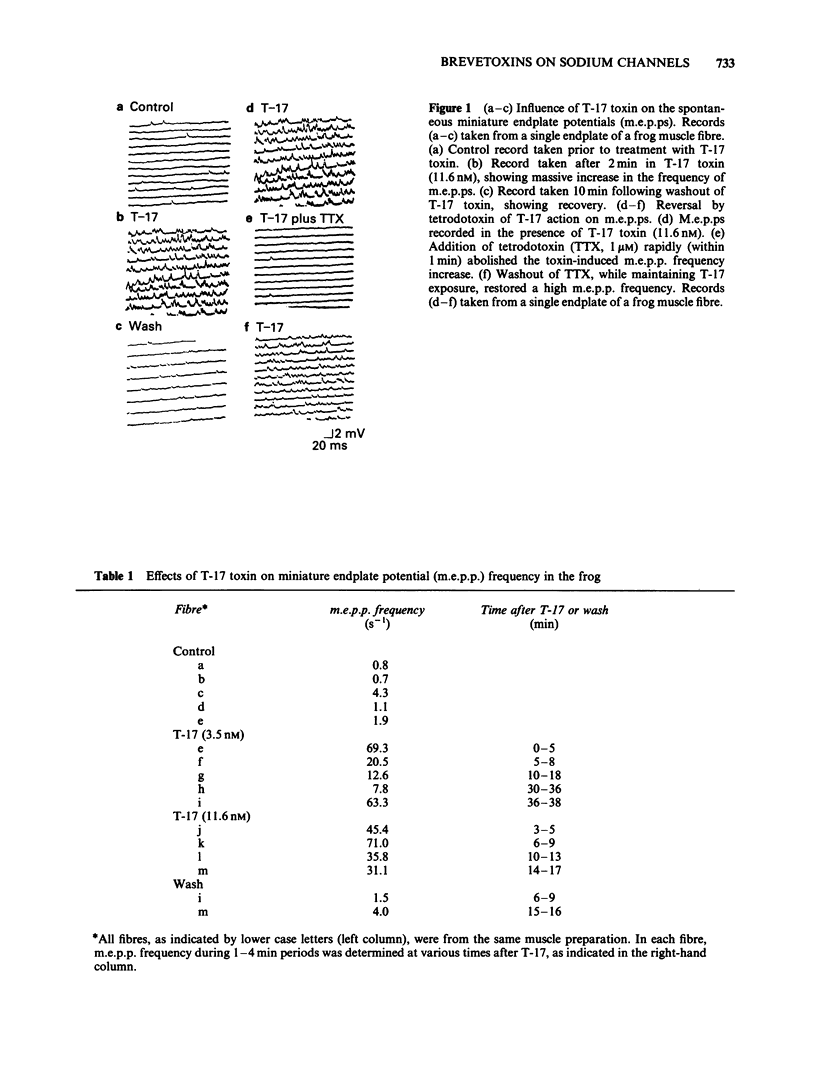
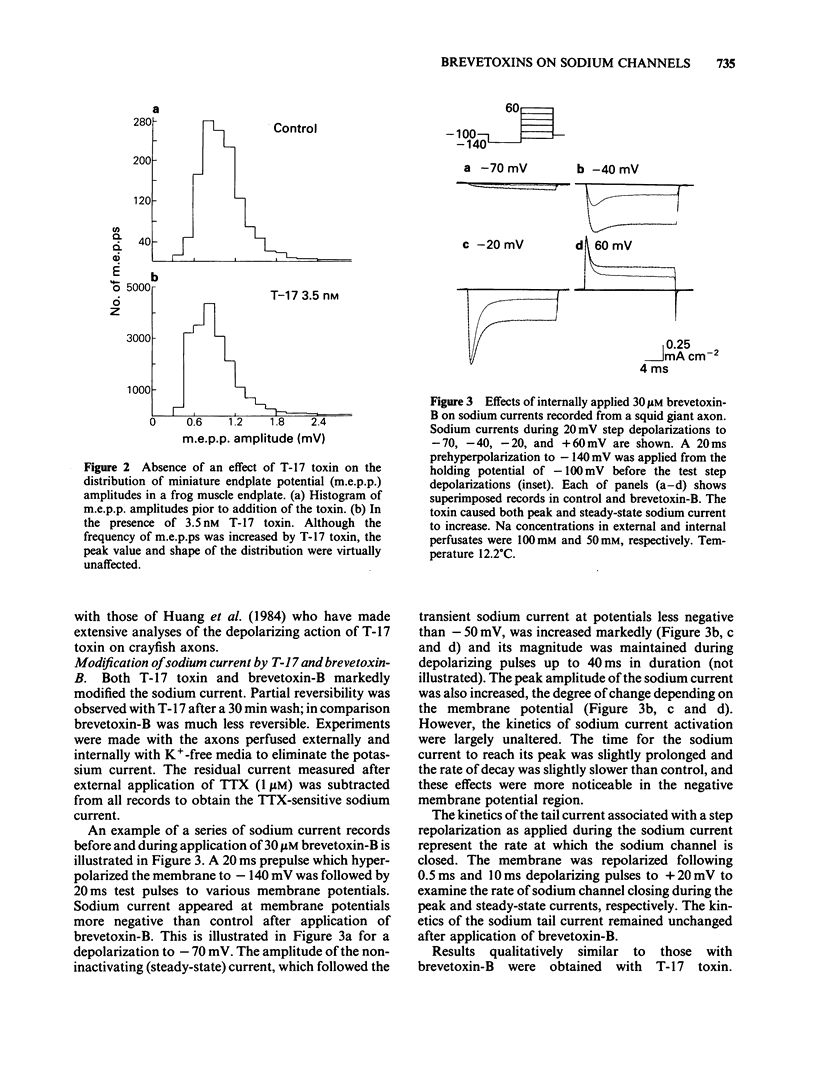
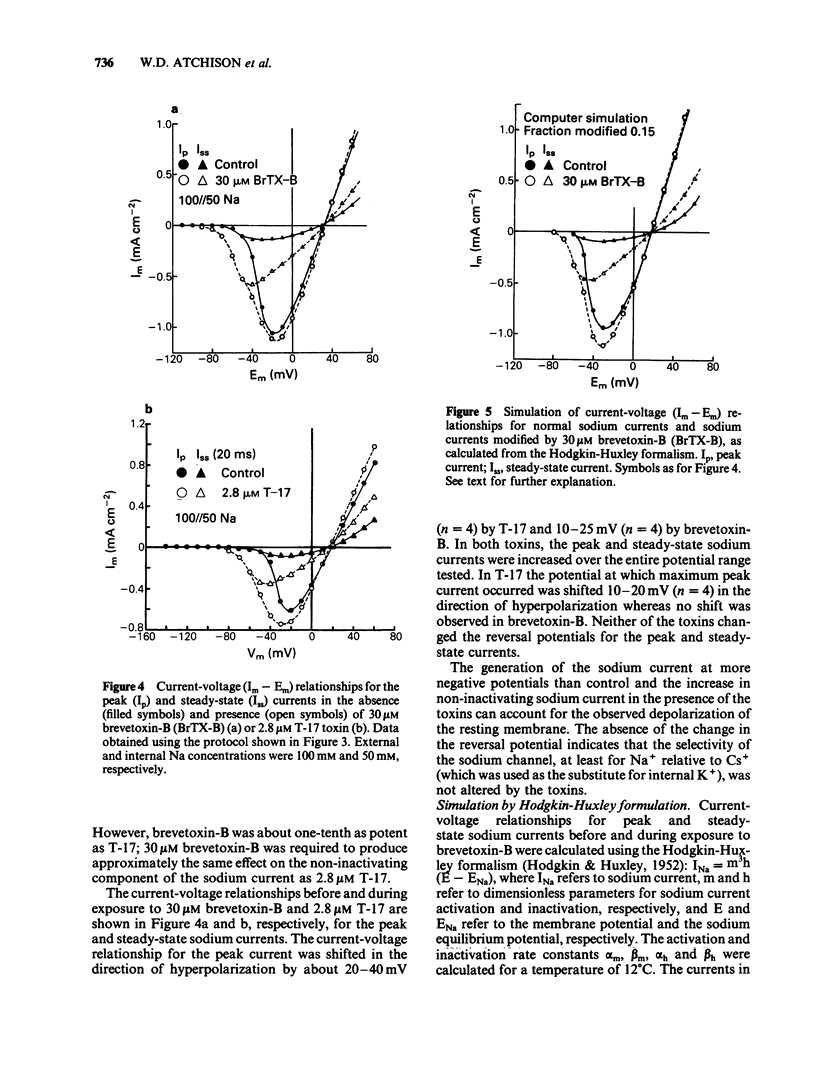
Actions of two structurally related toxins, T-17 and brevetoxin-B, isolated from the red-tide dinoflagellate, Ptychodiscus brevis, were studied on the giant axon of the squid and the neuromuscular junctions of the frog and rat. T-17 toxin caused a large increase in the frequency of miniature endplate potentials at nanomolar concentrations. In one typical case with a frog endplate, the frequency increased from 1.9 s-1 before application of 3.5 nM T-17 to 69.3 s-1 within 5 min after application. In the rat muscle, the mean frequency increased from 1.39 s-1 in control to 11.93 s-1 after application of 23.2 nM T-17. The increase in miniature endplate potential frequency was reversed by the addition of 1 microM tetrodotoxin, and was not observed in a solution containing elevated Mg2+ and reduced Ca2+ concentrations. External or internal application of T-17 toxin (2-5 microM) or brevetoxin-B (10-30 microM) to intact or internally perfused squid axons caused a depolarization of the membrane. This depolarization was abolished by the removal of external Na+ or by addition of tetrodotoxin to the external solution. In voltage clamped squid giant axons, exposure to T-17 toxin or brevetoxin-B increased the non-inactivating component of the tetrodotoxin-sensitive sodium current. The sodium current was activated at potentials 15 to 40 mV more negative than control. It is proposed that these toxins modify a fraction of the sodium channels to a form which opens at potentials more negative than normal and which inactivates to a lesser extent. This mechanism would predict a depolarization of the nerve membrane at the neuromuscular junction, thus explaining the increased discharge of transmitter.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden D. G., Bikhazi G., Decker S. J., Foldes F. F., Leung I. Neuromuscular blocking action of two brevetoxins from the Florida red tide organism Ptychodiscus brevis. Toxicon. 1984;22(1):75–84. doi: 10.1016/0041-0101(84)90140-5. [DOI] [PubMed] [Google Scholar]
- Baden D. G. Marine food-borne dinoflagellate toxins. Int Rev Cytol. 1983;82:99–150. doi: 10.1016/s0074-7696(08)60824-4. [DOI] [PubMed] [Google Scholar]
- Baden D. G., Mende T. J., Lichter W., Wellham L. Crystallization and toxicology of T34: a major toxin from Florida's red tide organism (Ptychodiscus brevis). Toxicon. 1981;19(4):455–462. doi: 10.1016/0041-0101(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Shinnick-Gallagher P. Effect of Gymnodinium breve toxin in the rat phrenic nerve diaphragm preparation. Br J Pharmacol. 1980 Jul;69(3):367–372. doi: 10.1111/j.1476-5381.1980.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. M., Wu C. H., Baden D. G. Depolarizing action of a red-tide dinoflagellate brevetoxin on axonal membranes. J Pharmacol Exp Ther. 1984 May;229(2):615–621. [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C. Mechanism of excitation block by the insecticide allethrin applied externally and internally to squid giant axons. Toxicol Appl Pharmacol. 1967 May;10(3):529–547. doi: 10.1016/0041-008x(67)90092-0. [DOI] [PubMed] [Google Scholar]
- Parmentier J. L., Narahashi T., Wilson W. A., Trieff N. M., Sadagopa Ramanujam V. M., Risk M. Electrophysiological and biochemical characteristics of Gymnodinium breve toxins. Toxicon. 1978;16(3):235–244. doi: 10.1016/0041-0101(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Risk M., Norris P. J., Coutinho-Netto J., Bradford H. F. Actions of Ptychodiscus brevis red tide toxin on metabolic and transmitter-releasing properties of synaptosomes. J Neurochem. 1982 Nov;39(5):1485–1488. doi: 10.1111/j.1471-4159.1982.tb12596.x. [DOI] [PubMed] [Google Scholar]
- Schmitt O., Schmidt H. Influence of calcium ions on the ionic currents of nodes of Ranvier treated with scorpion venom. Pflugers Arch. 1972;333(1):51–61. doi: 10.1007/BF00586041. [DOI] [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Modulation of sodium channels of squid nerve membranes by grayanotoxin I. J Pharmacol Exp Ther. 1981 Dec;219(3):614–624. [PubMed] [Google Scholar]
- Westerfield M., Moore J. W., Kim Y. S., Padilla G. M. How Gymnodinium breve red tide toxin(s) produces repetitive firing in squid axons. Am J Physiol. 1977 Jan;232(1):C23–C29. doi: 10.1152/ajpcell.1977.232.1.C23. [DOI] [PubMed] [Google Scholar]


