Abstract
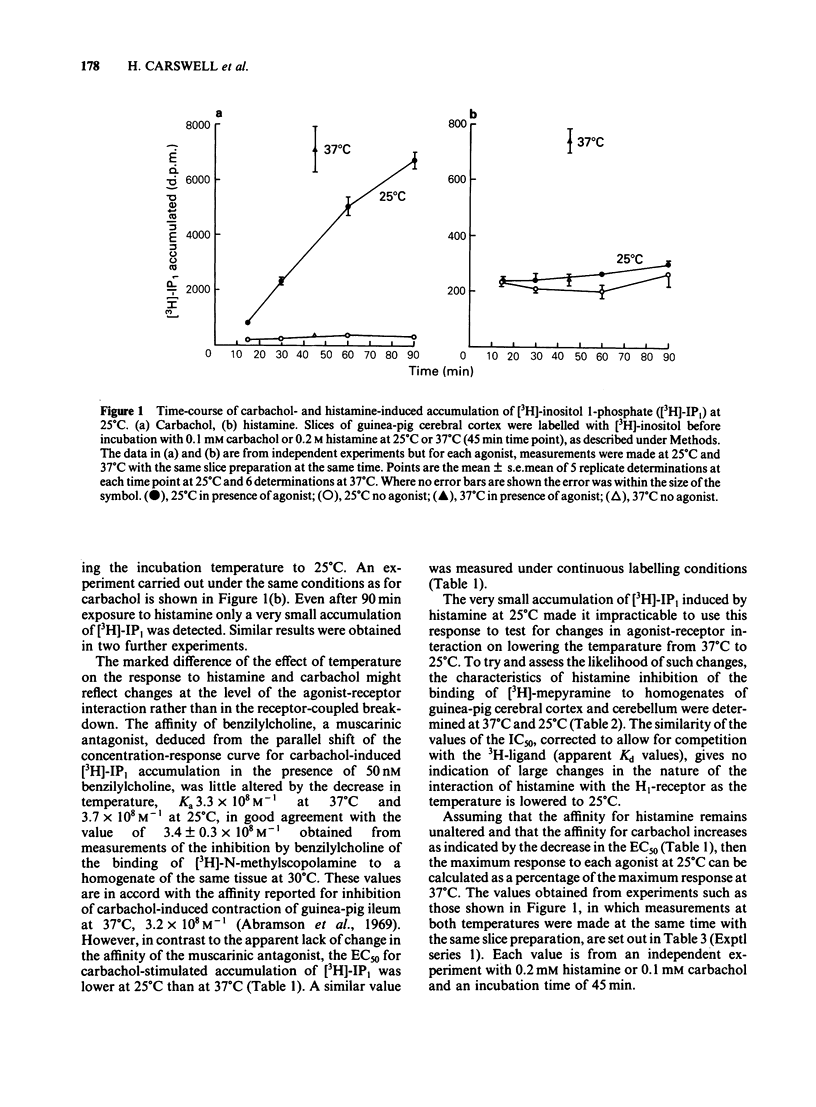
Slices of guinea-pig cerebral cortex were incubated with [3H]-inositol at 37 degrees C before exposure to histamine or carbachol at 37 degrees C or 25 degrees C. Histamine-stimulated accumulation of [3H]-inositol 1-phosphate ([3H]-IP1) at 25 degrees C was only 5-7% of that at 37 degrees C, whereas for carbachol the response at 25 degrees C was 45-49% of that at 37 degrees C. The affinity of benzilylcholine, obtained from inhibition of carbachol-induced accumulation of [3H]-IP1 was similar at 25 degrees C and 37 degrees C, but the EC50 for carbachol was lower at 25 degrees C (20 +/- 2 microM) than at 37 degrees C (42 +/- 2 microM). The IC50 for histamine inhibition of [3H]-mepyramine binding to homogenates of guinea-pig cerebral cortex did not differ significantly at 25 degrees C and 37 degrees C. Histamine-induced accumulations of [3H]-IP2 and [3H]-IP3 at 25 degrees C, expressed as a percentage of the accumulation at 37 degrees C, were also much less than the corresponding value for carbachol. These observations imply that the locus or pathway(s) of agonist-induced formation of [3H]-IP1 are not the same for histamine and carbachol.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson F. B., Barlow R. B., Mustafa M. G., Stephenson R. P. Relationships between chemical structure and affinity for acetylcholine receptors. Br J Pharmacol. 1969 Sep;37(1):207–233. doi: 10.1111/j.1476-5381.1969.tb09539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceves J., Mariscal S., Morrison K. E., Young J. M. The binding of doxepin to histamine H1-receptors in guinea-pig and rat brain. Br J Pharmacol. 1985 Feb;84(2):417–424. doi: 10.1111/j.1476-5381.1985.tb12925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bertaccini G., Zappia L. Evidence against the temperature-dependent interconversion of histamine H1- and H2-receptors in the guinea-pig ileum. Br J Pharmacol. 1983 Jan;78(1):11–16. doi: 10.1111/j.1476-5381.1983.tb09357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. A., Krueger C. A., Michalchuk A. Temperature and histamine receptor function--what is really happening? Can J Physiol Pharmacol. 1985 Jun;63(6):751–755. doi: 10.1139/y85-124. [DOI] [PubMed] [Google Scholar]
- Daum P. R., Downes C. P., Young J. M. Histamine stimulation of inositol 1-phosphate accumulation in lithium-treated slices from regions of guinea pig brain. J Neurochem. 1984 Jul;43(1):25–32. doi: 10.1111/j.1471-4159.1984.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced inositol phospholipid breakdown in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1985 Jun;85(2):499–512. doi: 10.1111/j.1476-5381.1985.tb08887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Bushfield M., Macphee C. H. Thyrotropin-releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumor cells. Studies with lithium. Mol Pharmacol. 1984 Mar;25(2):201–208. [PubMed] [Google Scholar]
- Eva C., Costa E. Potassium ion facilitation of phosphoinositide turnover activation by muscarinic receptor agonists in rat brain. J Neurochem. 1986 May;46(5):1429–1435. doi: 10.1111/j.1471-4159.1986.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Gorissen H., Aerts G., Laduron P. Characterization of digitonin-solubilized muscarinic receptor from rat brain. FEBS Lett. 1978 Dec 1;96(1):64–68. doi: 10.1016/0014-5793(78)81063-1. [DOI] [PubMed] [Google Scholar]
- Harrison R. W., Carswell H., Young J. M. Relative potencies of histamine H1-agonists on guinea-pig tracheal smooth muscle. Eur J Pharmacol. 1984 Nov 13;106(2):405–409. doi: 10.1016/0014-2999(84)90729-5. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Daly J. W. Accumulation of inositol phosphates and cyclic AMP in guinea-pig cerebral cortical preparations. Effects of norepinephrine, histamine, carbamylcholine and 2-chloroadenosine. Biochim Biophys Acta. 1985 Nov 20;847(2):207–216. doi: 10.1016/0167-4889(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., De la Cruz R. A., Daly J. W. Accumulations of inositol phosphates and cyclic AMP in brain slices: synergistic interactions of histamine and 2-chloroadenosine. Eur J Pharmacol. 1986 Mar 11;122(1):45–50. doi: 10.1016/0014-2999(86)90156-1. [DOI] [PubMed] [Google Scholar]
- Hurko O. Specific [3H]quinuclidinyl benzilate binding activity in digitonin-solubilized preparations from bovine brain. Arch Biochem Biophys. 1978 Oct;190(2):434–445. doi: 10.1016/0003-9861(78)90296-5. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Wallace R. M., Young J. M. Temperature dependence of the binding of [3H]mepyramine and related compounds to the histamine H1 receptor. Mol Pharmacol. 1983 Jan;23(1):60–66. [PubMed] [Google Scholar]
- Wilson D. B., Neufeld E. J., Majerus P. W. Phosphoinositide interconversion in thrombin-stimulated human platelets. J Biol Chem. 1985 Jan 25;260(2):1046–1051. [PubMed] [Google Scholar]


