Abstract
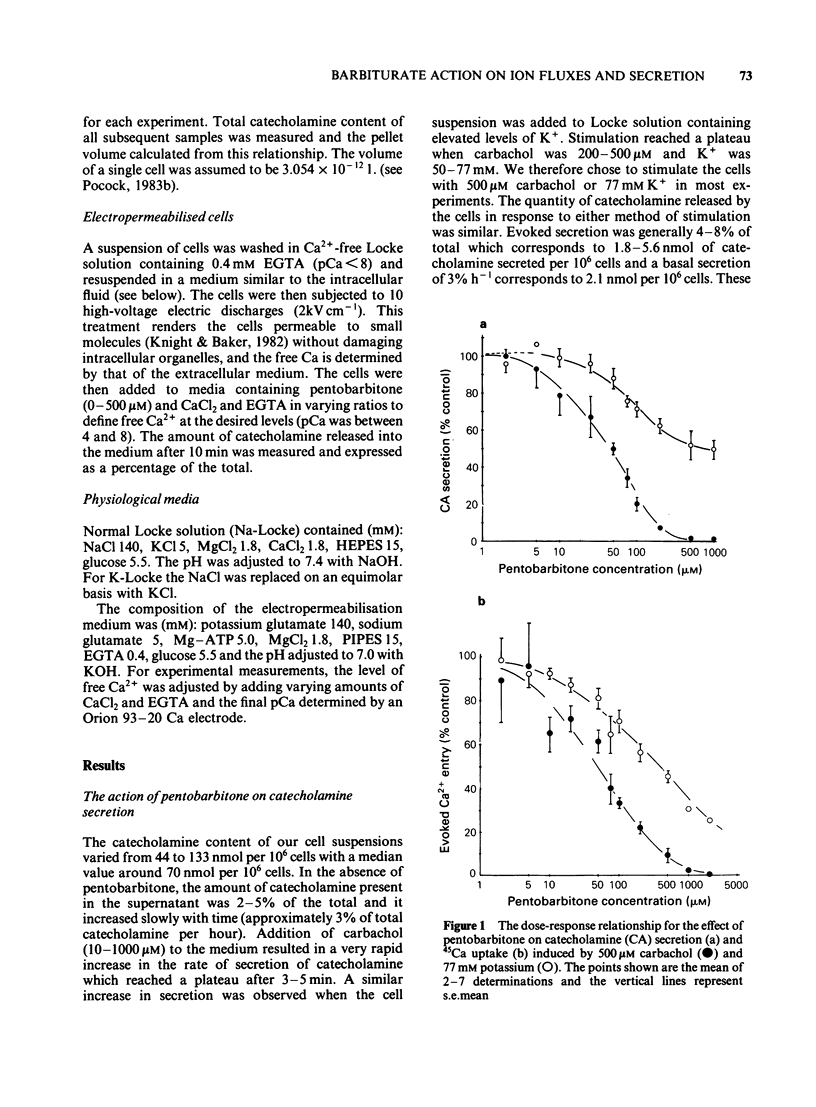
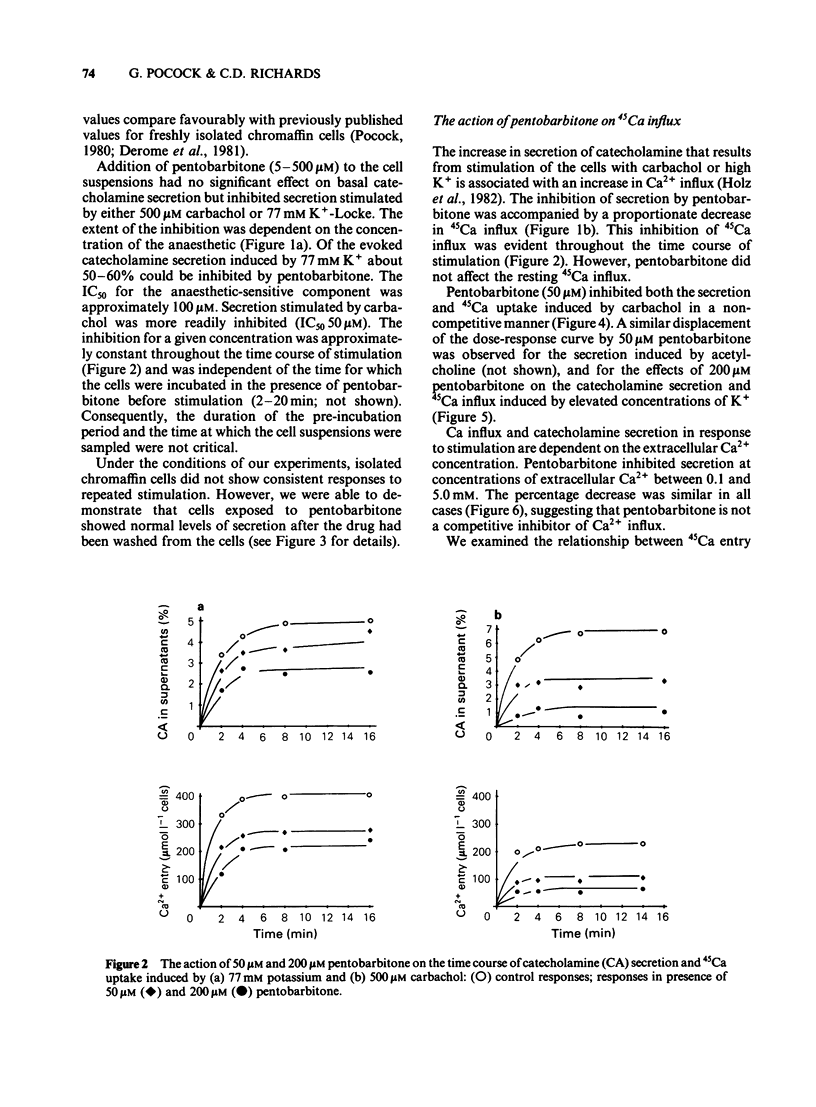
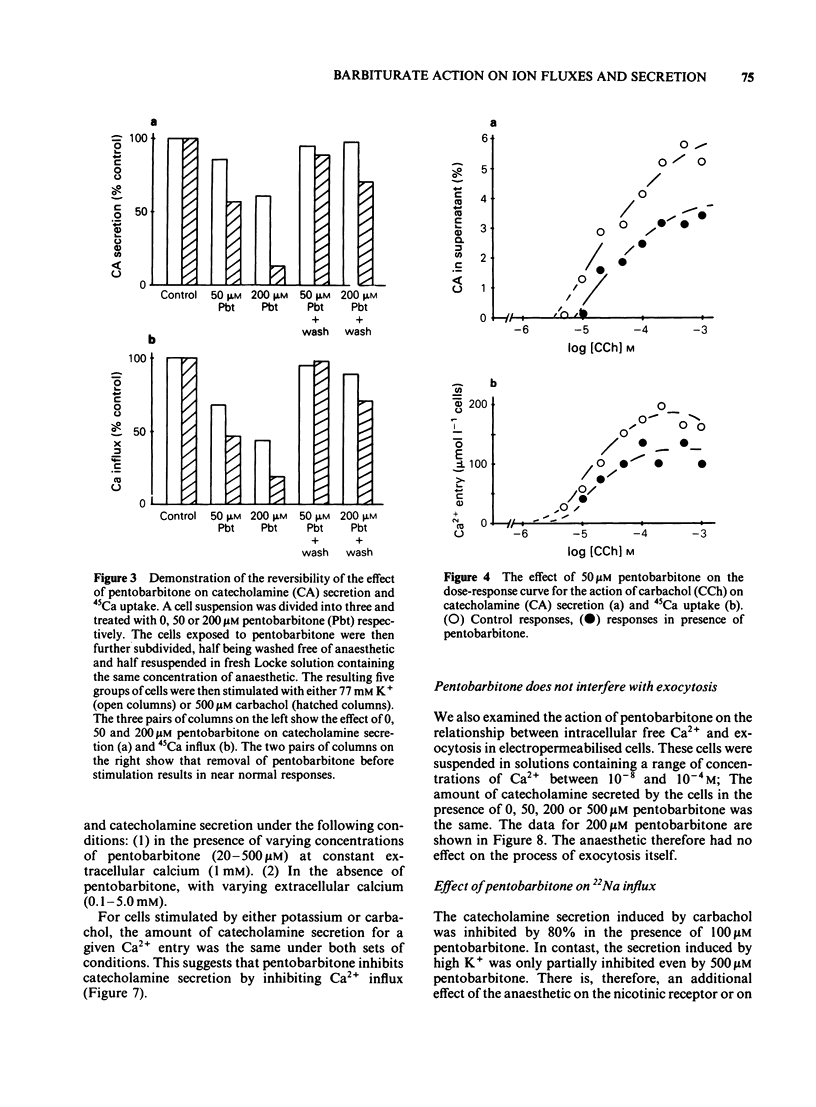
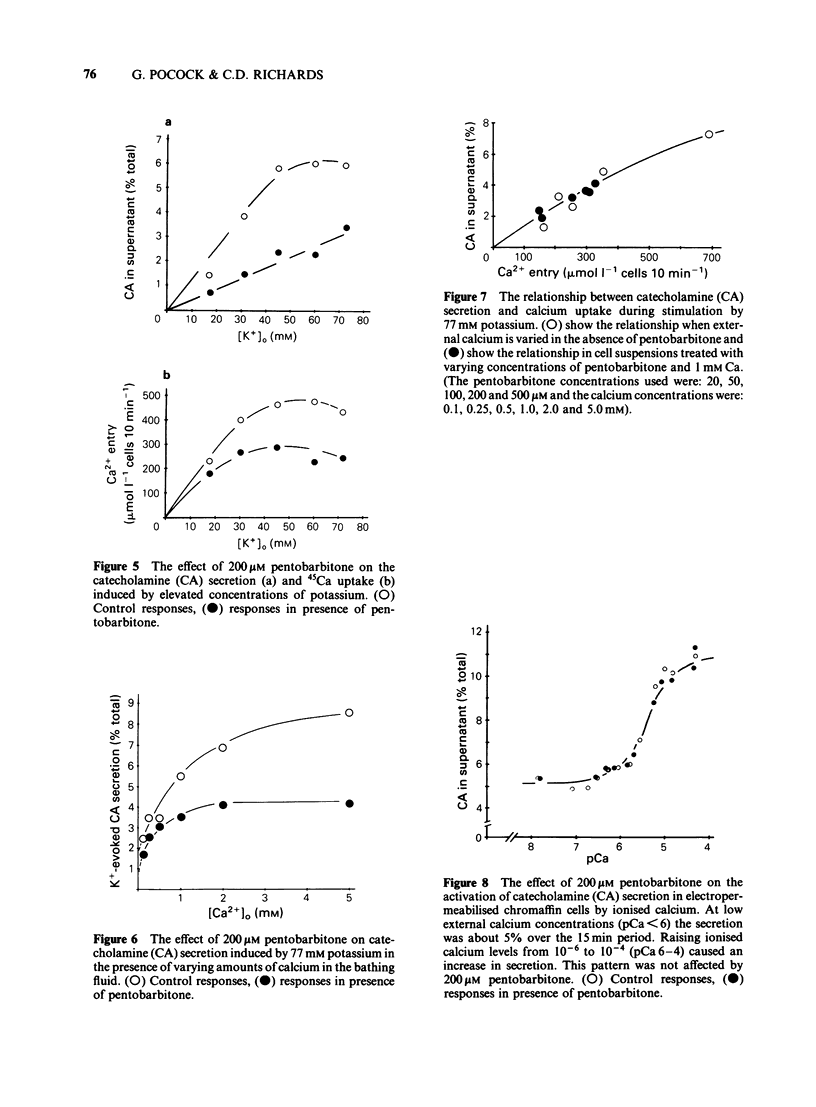
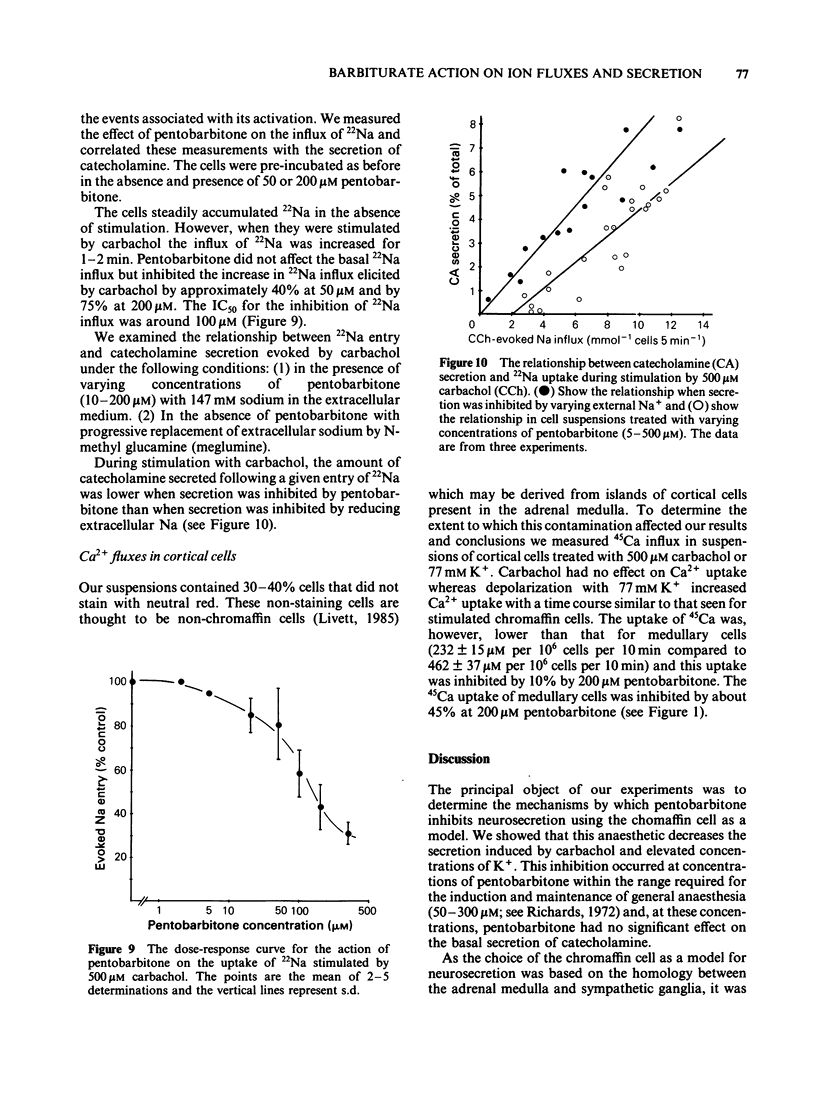
The action of pentobarbitone on stimulus-secretion coupling was studied in bovine isolated adrenal medullary cells. Pentobarbitone inhibited catecholamine release evoked by 500 microM carbachol with half maximal inhibition (IC50) around 50 microM. It also inhibited catecholamine release induced by depolarization with 77 mM potassium (IC50 100 microM). These effects of pentobarbitone were observed with concentrations that lie within the range encountered during general anaesthesia. Evoked secretion required the presence of calcium in the extracellular medium and was associated with an influx of Ca2+ through voltage-sensitive channels. Pentobarbitone inhibited 45Ca influx in response to both carbachol (IC50 50 microM) and K+-depolarization (IC50 150 microM). The action of pentobarbitone on the relationship between intracellular free Ca and exocytosis was examined using electropermeabilised cells which were suspended in solutions containing a range of concentrations of ionised calcium between 10(-8) and 10(-4)M. Catecholamine secretion was measured in the presence of 0, 50, 200 or 500 microM pentobarbitone. The anaesthetic had no effect on the activation of exocytosis by intracellular free calcium. When catecholamine secretion in response to potassium or carbachol was modulated by varying extracellular calcium or by adding pentobarbitone to the incubation medium, the amount of catecholamine secretion for a given Ca2+ entry was the same. Pentobarbitone inhibited the secretion and 45Ca uptake induced by carbachol in a non-competitive manner. The secretion evoked by nicotinic agonists was associated with an increase in 22Na influx. Pentobarbitone inhibited this influx with an IC50 of 100 microM. We concluded that: (a) Pentobarbitone inhibits the catecholamine secretion from bovine adrenal chromaffin cells induced by nicotinic agonists by non-competitive inhibition of the nicotinic receptor. (b) The decrease in Ca influx caused by pentobarbitone accounts fully for the decrease in secretion in response to depolarization with potassium.
Full text
PDF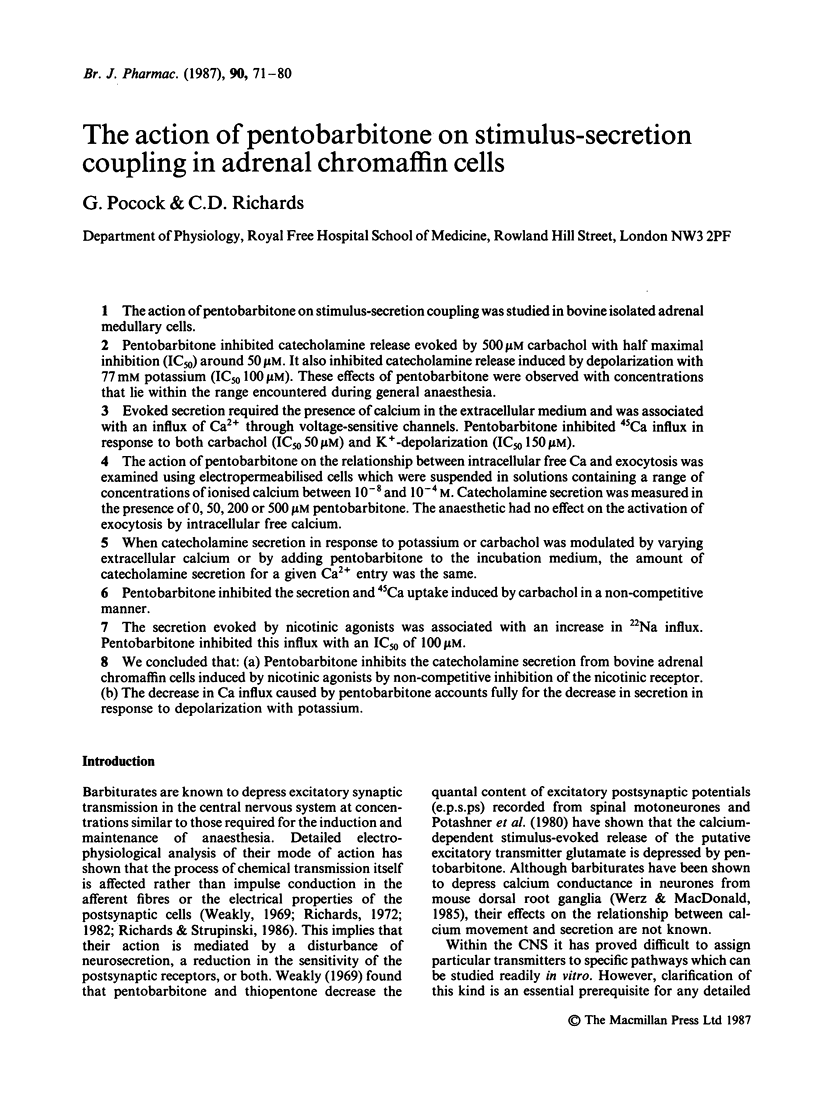




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley R. H., Brammer M. J., Marchbanks R. Measurement of intrasynaptosomal free calcium by using the fluorescent indicator quin-2. Biochem J. 1984 Apr 1;219(1):149–158. doi: 10.1042/bj2190149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Barbiturate inhibition of calcium uptake by depolarized nerve terminals in vitro. Mol Pharmacol. 1975 May;11(3):369–378. [PubMed] [Google Scholar]
- Bliss T. V., Richards C. D. Some experiments with in vitro hippocampal slices. J Physiol. 1971;214 (Suppl):7P–9P. [PubMed] [Google Scholar]
- Collins G. G. Release of endogenous amino acid neurotransmitter candidates from rat olfactory cortex slices: possible regulatory mechanisms and the effects of pentobarbitone. Brain Res. 1980 May 26;190(2):517–528. doi: 10.1016/0006-8993(80)90293-0. [DOI] [PubMed] [Google Scholar]
- Derome G., Tseng R., Mercier P., Lemaire I., Lemaire S. Possible muscarinic regulation of catecholamine secretion mediated by cyclic GMP in isolated bovine adrenal chromaffin cells. Biochem Pharmacol. 1981 Apr 15;30(8):855–860. doi: 10.1016/s0006-2952(81)80007-x. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McKinnon D. Effects of pentobarbitone on acetylcholine-activated channels in mammalian muscle. Br J Pharmacol. 1985 May;85(1):229–235. doi: 10.1111/j.1476-5381.1985.tb08851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A., Frye R. A. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982 Sep;39(3):635–646. doi: 10.1111/j.1471-4159.1982.tb07940.x. [DOI] [PubMed] [Google Scholar]
- Kataoka Y., Gutman Y., Guidotti A., Panula P., Wroblewski J., Cosenza-Murphy D., Wu J. Y., Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall T. J., Minchin M. C. The effects of anaesthetics on the uptake and release of amino acid neurotransmitters in thalamic slices. Br J Pharmacol. 1982 Jan;75(1):219–227. doi: 10.1111/j.1476-5381.1982.tb08776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Kesteven N. T. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- MATTHEWS E. K., QUILLIAM J. P. EFFECTS OF CENTRAL DEPRESSANT DRUGS UPON ACETYLCHOLINE RELEASE. Br J Pharmacol Chemother. 1964 Apr;22:415–440. doi: 10.1111/j.1476-5381.1964.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin M. C. The effect of anaesthetics on the uptake and release of gamma-aminobutyrate and D-aspartate in rat brain slices. Br J Pharmacol. 1981 Jul;73(3):681–689. doi: 10.1111/j.1476-5381.1981.tb16803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Iwamoto E. T. Action of pentobarbital on sympathetic ganglion cells. J Neurophysiol. 1978 Jul;41(4):977–986. doi: 10.1152/jn.1978.41.4.977. [DOI] [PubMed] [Google Scholar]
- Pocock G. Ion movements in isolated bovine adrenal medullary cells treated with ouabain. Mol Pharmacol. 1983 May;23(3):681–697. [PubMed] [Google Scholar]
- Pocock G. Ionic and metabolic requirements for stimulation of secretion by ouabain in bovine adrenal medullary cells. Mol Pharmacol. 1983 May;23(3):671–680. [PubMed] [Google Scholar]
- Richards C. D., Metcalfe J. C., Smith G. A., Hesketh T. R. Changes in free-calcium levels and pH in synaptosomes during transmitter release. Biochim Biophys Acta. 1984 Apr 16;803(4):215–220. doi: 10.1016/0167-4889(84)90110-1. [DOI] [PubMed] [Google Scholar]
- Richards C. D. On the mechanism of barbiturate anaesthesia. J Physiol. 1972 Dec;227(3):749–767. doi: 10.1113/jphysiol.1972.sp010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Smaje J. C. Anaesthetics depress the sensitivity of cortical neurones to L-glutamate. Br J Pharmacol. 1976 Nov;58(3):347–357. [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Strupinski K. An analysis of the action of pentobarbitone on the excitatory postsynaptic potentials and membrane properties of neurones in the guinea-pig olfactory cortex. Br J Pharmacol. 1986 Oct;89(2):321–325. doi: 10.1111/j.1476-5381.1986.tb10263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. The actions of pentobarbitone, procaine and tetrodotoxin on synaptic transmission in the olfactory cortex of the guinea-pig. Br J Pharmacol. 1982 Apr;75(4):639–646. doi: 10.1111/j.1476-5381.1982.tb09185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S., Yamamoto C. Blocking action of pentobarbital on receptors for excitatory amino acids in the guinea pig hippocampus. Exp Brain Res. 1985;59(2):226–231. doi: 10.1007/BF00230901. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Herz R., Rosenheck K. Stimulus-secretion coupling in chromaffin cells isolated from bovine adrenal medulla. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5036–5040. doi: 10.1073/pnas.74.11.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Barbiturates decrease voltage-dependent calcium conductance of mouse neurons in dissociated cell culture. Mol Pharmacol. 1985 Sep;28(3):269–277. [PubMed] [Google Scholar]


