Abstract
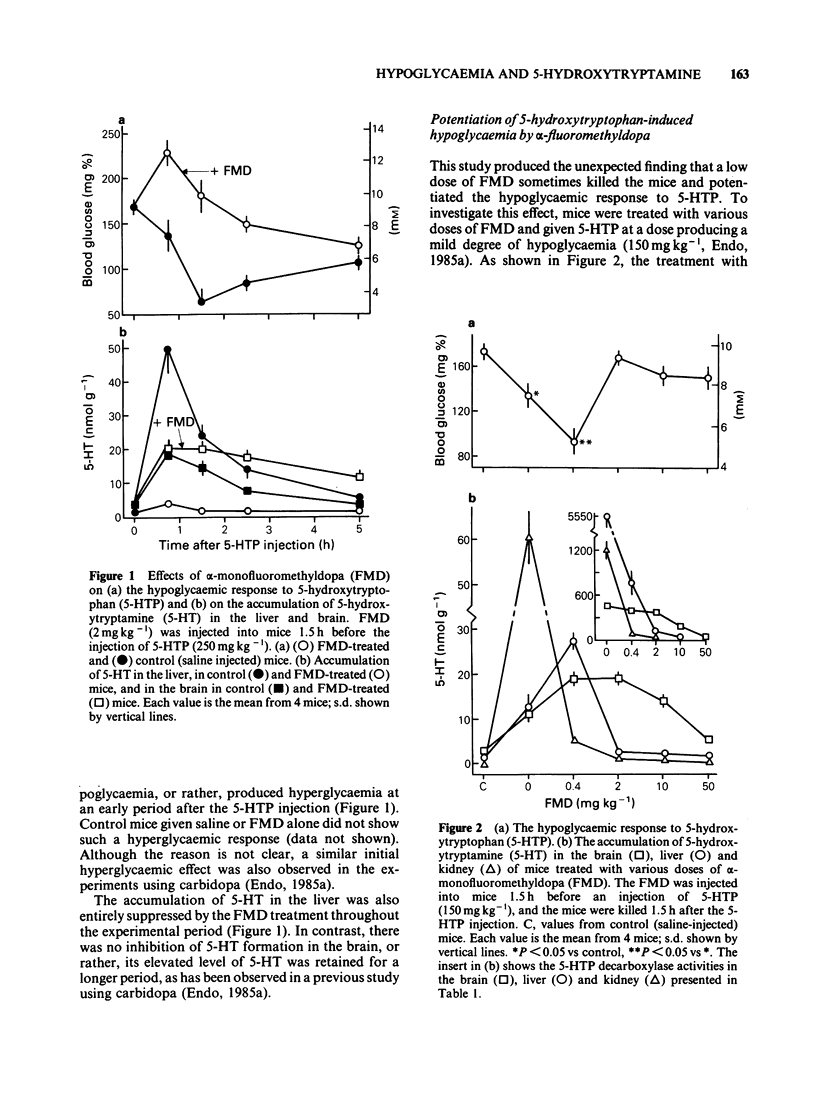
Experiments were done to examine whether the accumulation of 5-hydroxytryptamine (5-HT) in the liver is responsible for the hypoglycaemia induced in mice by 5-hydroxytryptophan (5-HTP) and lipopolysaccharides (LPS). (+/-)-alpha-Monofluoromethyldopa (FMD), a potent irreversible inhibitor of aromatic amino acid decarboxylase, suppressed the 5-HTP-induced accumulation of 5-HT in the liver at a dose of 2 mg kg-1 or more, but potentiated the accumulation at lower dose of 0.4 mg kg-1. Corresponding to these effects, the hypoglycaemic response was prevented by the higher doses of FMD and potentiated by the lower dose. These contrasting effects of FMD were explicable by the amounts of 5-HTP entering the liver. In contrast, FMD did not prevent either the hypoglycaemia or the accumulation of 5-HT in the liver induced by LPS. These results further support the hypothesis that the accumulation of 5-HT in the liver is causally related to the hypoglycaemia induced by 5-HTP and indicate that the LPS-induced 5-HT accumulation in the liver is not derived from stimulation of 5-HT synthesis. It is still not clear whether the accumulation of 5-HT in the liver is involved in the hypoglycaemic response to LPS.
Full text
PDF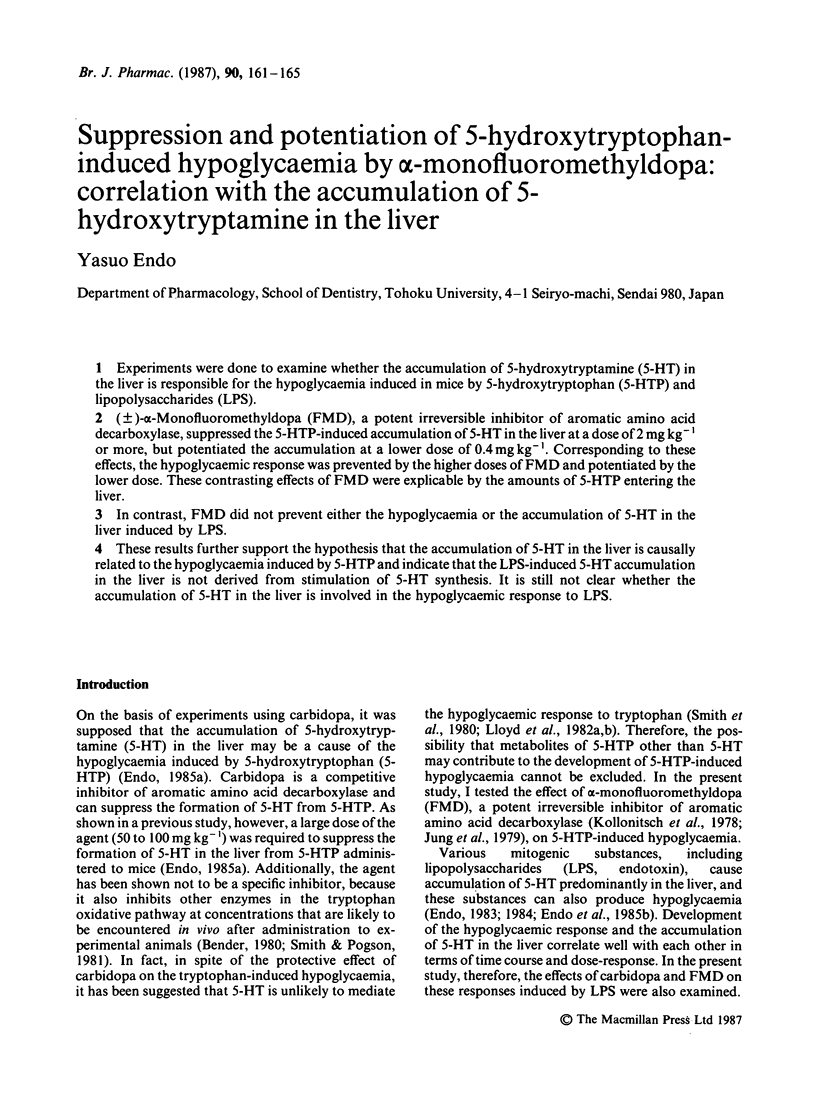



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DiTullio N. W., Berkoff C. E., Blank B., Kostos V., Stack E. J., Saunders H. L. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem J. 1974 Mar;138(3):387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y. A lipopolysaccharide and concanavalin A induce variations of serotonin levels in mouse tissues. Eur J Pharmacol. 1983 Aug 5;91(4):493–499. doi: 10.1016/0014-2999(83)90175-9. [DOI] [PubMed] [Google Scholar]
- Endo Y. Evidence that the accumulation of 5-hydroxytryptamine in the liver but not in the brain may cause the hypoglycaemia induced by 5-hydroxytryptophan. Br J Pharmacol. 1985 Jul;85(3):591–598. doi: 10.1111/j.1476-5381.1985.tb10553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y. Induction of hypoglycaemia and accumulation of 5-hydroxytryptamine in the liver after the injection of mitogenic substances into mice. Br J Pharmacol. 1984 Apr;81(4):645–650. doi: 10.1111/j.1476-5381.1984.tb16130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins J. P., Cornell R. P. Depression of hepatic gluconeogenesis and the hypoglycemia of endotoxin shock. Am J Physiol. 1974 Oct;227(4):778–781. doi: 10.1152/ajplegacy.1974.227.4.778. [DOI] [PubMed] [Google Scholar]
- Jomain-Baum M., Schramm V. L., Hanson R. W. Mechanism of 3-mercaptopicolinic acid inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1976 Jan 10;251(1):37–44. [PubMed] [Google Scholar]
- Jung M. J., Palfreyman M. G., Wagner J., Bey P., Ribereau-Gayon G., Zraïka M., Koch-Weser J. Inhibition of monoamine synthesis by irreversible blockade of aromatic aminoacid decarboxylase with alpha-monofluoromethyldopa. Life Sci. 1979 Mar 12;24(11):1037–1042. doi: 10.1016/0024-3205(79)90324-2. [DOI] [PubMed] [Google Scholar]
- Kollonitsch J., Perkins L. M., Patchett A. A., Doldouras G. A., Marburg S., Duggan D. E., Maycock A. L., Aster S. D. Selective inhibitors of biosynthesis of aminergic neurotransmitters. Nature. 1978 Aug 31;274(5674):906–908. doi: 10.1038/274906a0. [DOI] [PubMed] [Google Scholar]
- Lernmark A. The significance of 5-hydroxytryptamine for insulin secretion in the mouse. Horm Metab Res. 1971 Sep;3(5):305–309. doi: 10.1055/s-0028-1094131. [DOI] [PubMed] [Google Scholar]
- Lloyd P., Smith S. A., Stribling D., Pogson C. I. Factors affecting tryptophan-induced hypoglycaemia in rats. Biochem Pharmacol. 1982 Nov 15;31(22):3563–3569. doi: 10.1016/0006-2952(82)90576-7. [DOI] [PubMed] [Google Scholar]
- Lloyd P., Stribling D., Pogson C. I. Endotoxin and tryptophan-induced hypoglycaemia in rats. Biochem Pharmacol. 1982 Nov 15;31(22):3571–3576. doi: 10.1016/0006-2952(82)90577-9. [DOI] [PubMed] [Google Scholar]
- McCallum R. E., Berry L. J. Effects of endotoxin on gluconeogenesis, glycogen synthesis, and liver glycogen synthase in mice. Infect Immun. 1973 Apr;7(4):642–654. doi: 10.1128/iai.7.4.642-654.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Carr F. P., Pogson C. I. The metabolism of L-tryptophan by isolated rat liver cells. Quantification of the relative importance of, and the effect of nutritional status on, the individual pathways of tryptophan metabolism. Biochem J. 1980 Nov 15;192(2):673–686. doi: 10.1042/bj1920673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Inhibition of hepatic gluconeogenesis by tryptophan metabolites in rats and guinea pigs. Biochem Pharmacol. 1979 Jul 15;28(14):2145–2148. doi: 10.1016/0006-2952(79)90196-5. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Pogson C. I. Effects of benserazide and carbidopa on the metabolism of L-tryptophan by isolated rat liver cells. Biochem Pharmacol. 1981 Mar 15;30(6):623–628. doi: 10.1016/0006-2952(81)90135-0. [DOI] [PubMed] [Google Scholar]
- Tadano T., Endo Y., Kisara K. A simple determination of serotonin, 5-hydroxyindoleacetic acid and 5-hydroxytryptophan decarboxylase activity in rat brain areas and parallel correlation among the levels. Jpn J Pharmacol. 1980 Jun;30(3):347–356. doi: 10.1254/jjp.30.347. [DOI] [PubMed] [Google Scholar]


