Abstract
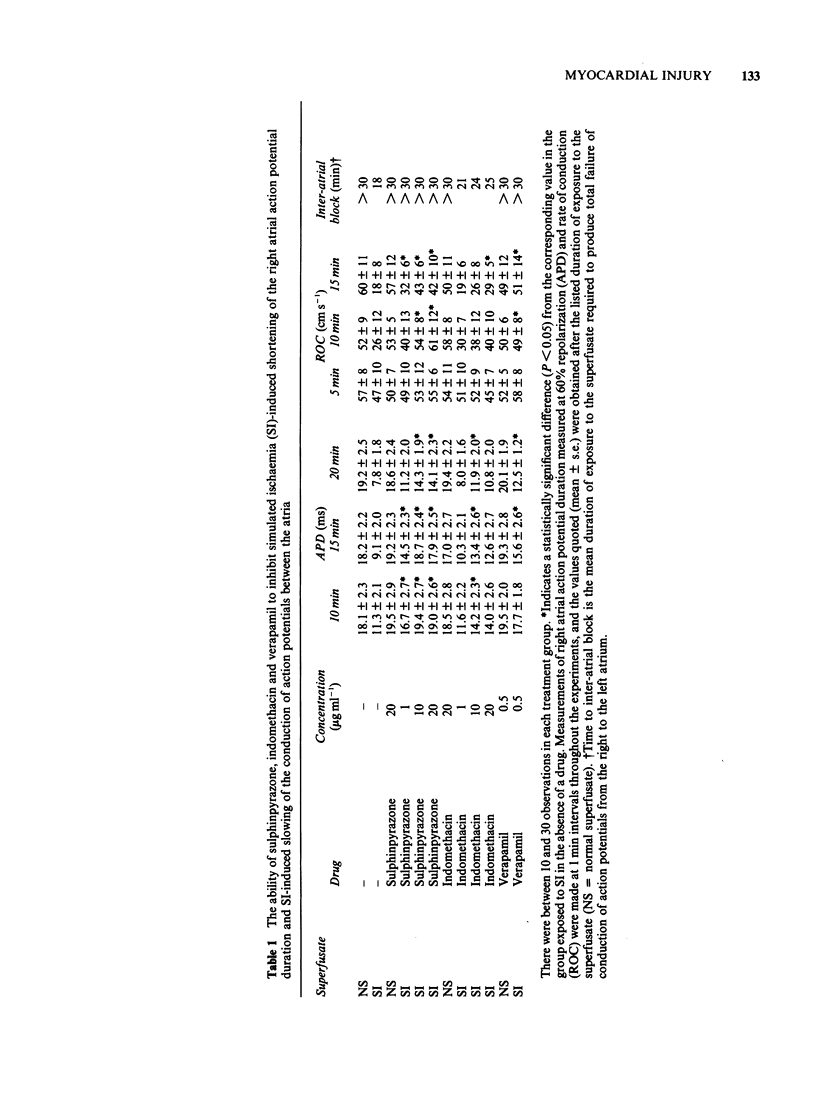
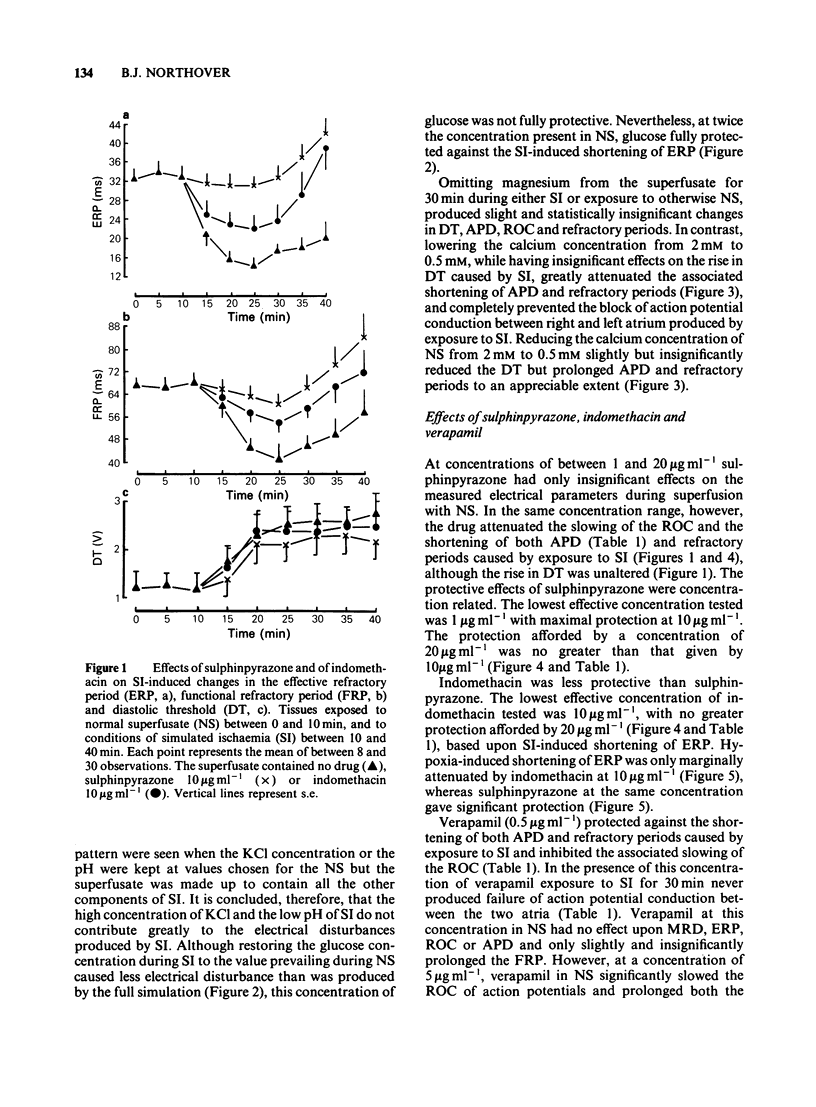
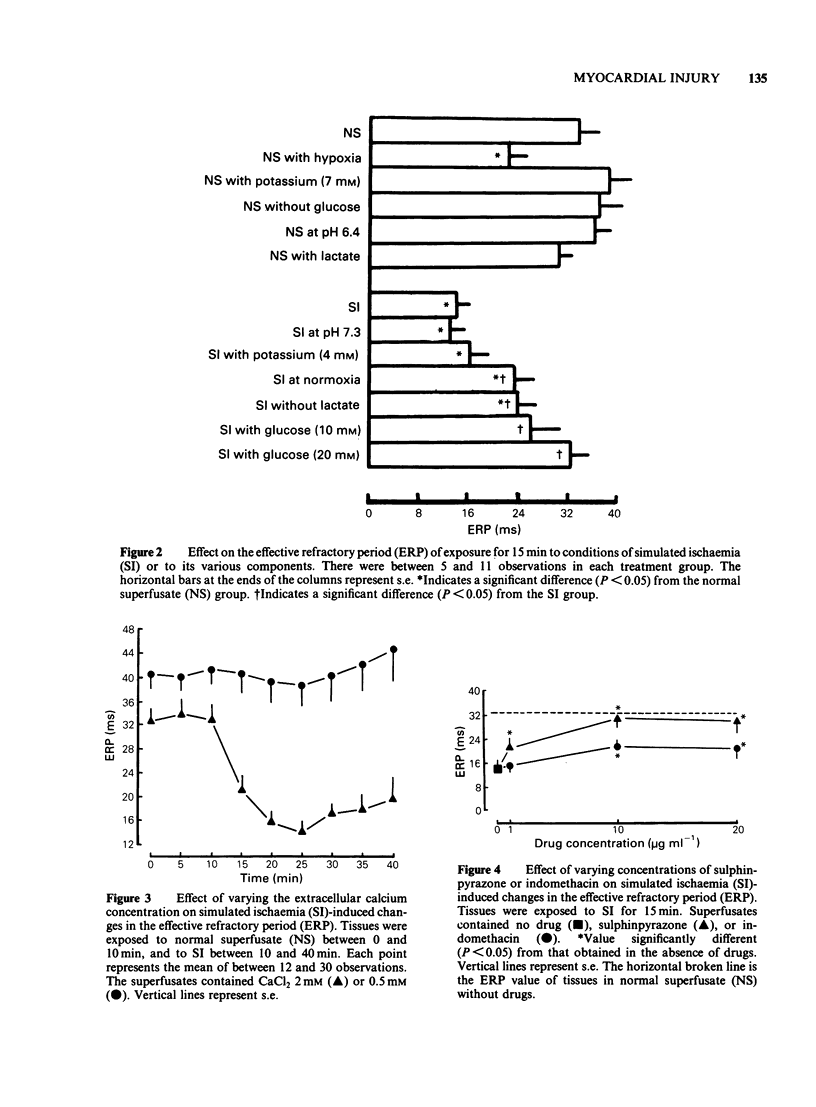
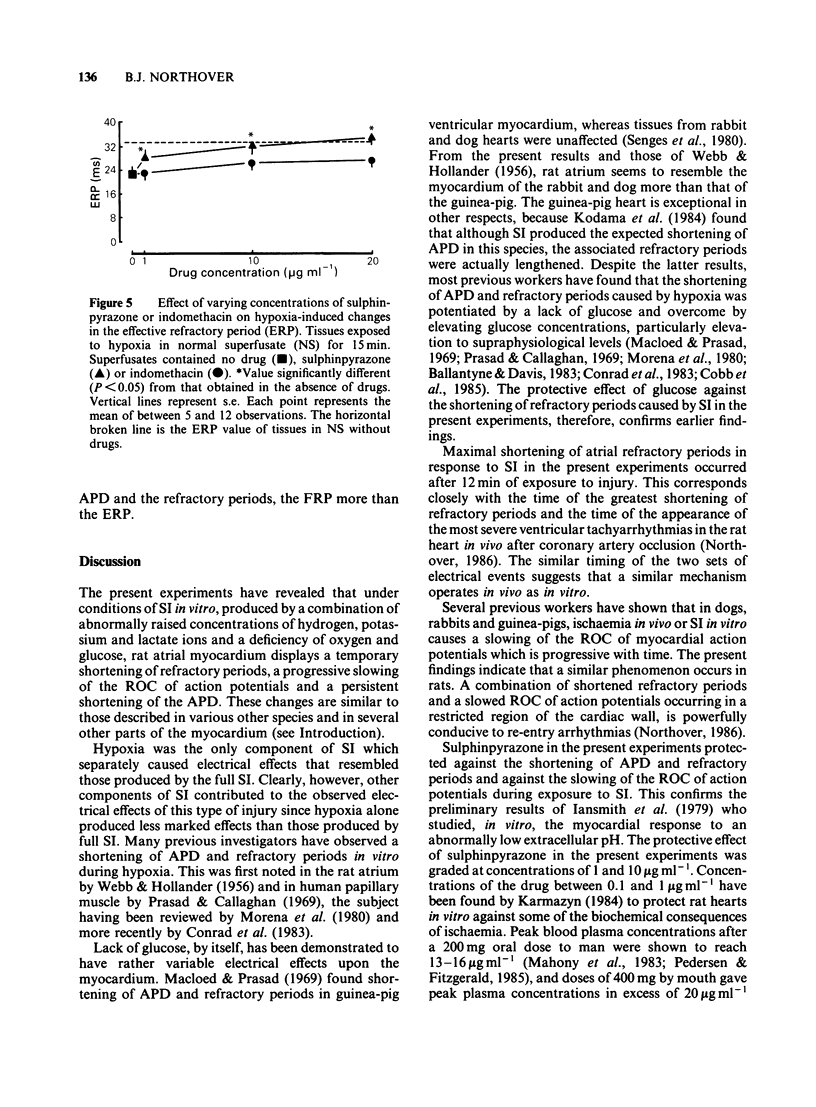
Glass microelectrodes were used to record intracellular electrical activity from rat isolated and superfused atrial myocardium during external electrical stimulation. After 2 h in normal oxygenated physiological salt solution the muscle was exposed for 30 min to a superfusate simulating the composition of extracellular fluid during myocardial ischaemia (SI). This fluid contained lactate (20 mM), a raised potassium concentration (7 mM), no glucose and a pH lowered to 6.4, and was gassed with N2 in place of O2 (hypoxia). During SI the diastolic threshold voltage for stimulation increased, the speed of action potential conduction between the right and left atria slowed, and both the effective and functional refractory periods of the right atrium shortened, as did the duration of the right atrial action potential. The only component of SI which separately caused electrical changes similar to those of the full simulation was hypoxia. Addition to the superfusate of verapamil (0.5 micrograms ml-1), sulphinpyrazone (1-20 micrograms ml-1) or indomethacin (10-20 micrograms ml-1) attenuated many of the SI-induced electrical changes, although indomethacin was much less effective than the other two drugs. Lowering the calcium concentration of the superfusate from 2 mM to 0.5 mM protected against the SI-induced electrical changes that were inhibitable with sulphinpyrazone and verapamil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anno T., Kodama I., Shibata S., Toyama J., Yamada K. Effects of calcium, calcium entry blockers and calmodulin inhibitors on atrioventricular conduction disturbances induced by hypoxia. Br J Pharmacol. 1986 May;88(1):277–284. doi: 10.1111/j.1476-5381.1986.tb09496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne F., 3rd, Davis L. D. Effects of glucose on the transmembrane potential of hypoxic canine ventricular muscle fibers. Am Heart J. 1983 Oct;106(4 Pt 1):670–673. doi: 10.1016/0002-8703(83)90085-6. [DOI] [PubMed] [Google Scholar]
- Cobbe S. M., Manley B. S., Alexopoulos D. Interaction of the effects of hypoxia, substrate depletion, acidosis and hyperkalaemia on the class III antiarrhythmic properties of sotalol. Cardiovasc Res. 1985 Nov;19(11):668–673. doi: 10.1093/cvr/19.11.668. [DOI] [PubMed] [Google Scholar]
- Conrad C. H., Mark R. G., Bing O. H. Outward current and repolarization in hypoxic rat myocardium. Am J Physiol. 1983 Mar;244(3):H341–H350. doi: 10.1152/ajpheart.1983.244.3.H341. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Cell-to-cell communication in heart and other tissues. Prog Biophys Mol Biol. 1982;39(3):147–182. doi: 10.1016/0079-6107(83)90016-0. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Effect of intracellular injection of calcium and strontium on cell communication in heart. J Physiol. 1975 Sep;250(2):231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar E., Janse M. J., Durrer D. The effect of "ischemic" blood on transmembrane potentials of normal porcine ventricular myocardium. Circulation. 1977 Mar;55(3):455–462. doi: 10.1161/01.cir.55.3.455. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R., Moffat M. P., Lukas A. Possible mechanisms of ventricular arrhythmias elicited by ischemia followed by reperfusion. Studies on isolated canine ventricular tissues. Circ Res. 1985 Feb;56(2):184–194. doi: 10.1161/01.res.56.2.184. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. B., Karmazyn M. Comparative effects of calcium channel blocking agents and varying extracellular calcium concentration on hypoxia/reoxygenation and ischemia/reperfusion-induced cardiac injury. J Pharmacol Exp Ther. 1984 Mar;228(3):761–768. [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLANDER P. B., WEBB J. L. Metabolic aspects of the relationship between the contractility and membrane potentials of the rat atrium. Circ Res. 1956 Sep;4(5):618–626. doi: 10.1161/01.res.4.5.618. [DOI] [PubMed] [Google Scholar]
- Karmazyn M. A direct protective effect of sulphinpyrazone on ischaemic and reperfused rat hearts. Br J Pharmacol. 1984 Sep;83(1):221–226. doi: 10.1111/j.1476-5381.1984.tb10138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Nakaya H., Kanno M. Effects of verapamil and lidocaine on changes in action potential characteristics and conduction time induced by combined hypoxia, hyperkalemia, and acidosis in canine ventricular myocardium. J Cardiovasc Pharmacol. 1982 Jul-Aug;4(4):658–667. doi: 10.1097/00005344-198207000-00019. [DOI] [PubMed] [Google Scholar]
- Kimura S., Nakaya H., Kanno M. Electrophysiological effects of diltiazem, nifedipine and Ni2+ on the subepicardial muscle cells of canine heart under the condition of combined hypoxia, hyperkalemia and acidosis. Naunyn Schmiedebergs Arch Pharmacol. 1983 Nov;324(3):228–232. doi: 10.1007/BF00503900. [DOI] [PubMed] [Google Scholar]
- Kodama I., Wilde A., Janse M. J., Durrer D., Yamada K. Combined effects of hypoxia, hyperkalemia and acidosis on membrane action potential and excitability of guinea-pig ventricular muscle. J Mol Cell Cardiol. 1984 Mar;16(3):247–259. doi: 10.1016/s0022-2828(84)80591-x. [DOI] [PubMed] [Google Scholar]
- MacLeod D. P., Prasad K. Influence of glucose on the transmembrane action potential of papillary muscle. Effects of concentration, phlorizin and insulin, nonmetabolizable sugars, and stimulators of glycolysis. J Gen Physiol. 1969 Jun;53(6):792–815. doi: 10.1085/jgp.53.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony C., Wolfram K. M., Nash P. V., Bjornsson T. D. Kinetics and metabolism of sulfinpyrazone. Clin Pharmacol Ther. 1983 Apr;33(4):491–497. doi: 10.1038/clpt.1983.67. [DOI] [PubMed] [Google Scholar]
- Moréna H., Janse M. J., Fiolet J. W., Krieger W. J., Crijns H., Durrer D. Comparison of the effects of regional ischemia, hypoxia, hyperkalemia, and acidosis on intracellular and extracellular potentials and metabolism in the isolated porcine heart. Circ Res. 1980 May;46(5):634–646. doi: 10.1161/01.res.46.5.634. [DOI] [PubMed] [Google Scholar]
- Nakaya H., Hattori Y., Kanno M. Effects of calcium antagonists and lidocaine on conduction delay induced by acute myocardial ischemia in dogs. Jpn J Pharmacol. 1980 Oct;30(5):587–597. doi: 10.1254/jjp.30.587. [DOI] [PubMed] [Google Scholar]
- Nakaya H., Kanno M. Effects of verapamil on conduction delay and potassium efflux induced by global ischemia in isolated rabbit hearts. Eur J Pharmacol. 1982 Apr 23;79(3-4):185–192. doi: 10.1016/0014-2999(82)90624-0. [DOI] [PubMed] [Google Scholar]
- Northover B. J. A protective effect of sulphinpyrazone against coronary occlusion-induced shortening of myocardial refractory periods in the rat. Br J Pharmacol. 1986 May;88(1):141–148. doi: 10.1111/j.1476-5381.1986.tb09480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A. K., FitzGerald G. A. Cyclooxygenase inhibition, platelet function, and metabolite formation during chronic sulfinpyrazone dosing. Clin Pharmacol Ther. 1985 Jan;37(1):36–42. doi: 10.1038/clpt.1985.8. [DOI] [PubMed] [Google Scholar]
- Prasad K., Callaghan J. C. Effects of glucose metabolism on the transmembrane action potential and contraction of human papillary muscle during surgical anoxia. Ann Thorac Surg. 1969 Jun;7(6):571–581. doi: 10.1016/s0003-4975(10)66397-7. [DOI] [PubMed] [Google Scholar]
- Rosenkranz B., Fischer C., Jakobsen P., Kirstein Pedersen A., Frölich J. C. Plasma levels of sulfinpyrazone and of two of its metabolites after a single dose and during the steady state. Eur J Clin Pharmacol. 1983;24(2):231–235. doi: 10.1007/BF00613823. [DOI] [PubMed] [Google Scholar]
- Rothermich N. O. An extended study of indomethacin. I. Clinical pharmacology. JAMA. 1966 Feb 14;195(7):531–536. [PubMed] [Google Scholar]
- Senges J., Brachmann J., Pelzer D., Krämer B., Kübler W. Combined effects of glucose and hypoxia on cardiac automaticity and conduction. J Mol Cell Cardiol. 1980 Mar;12(3):311–323. doi: 10.1016/0022-2828(80)90043-7. [DOI] [PubMed] [Google Scholar]
- Vleugels A., Vereecke J., Carmeliet E. Ionic currents during hypoxia in voltage-clamped cat ventricular muscle. Circ Res. 1980 Oct;47(4):501–508. doi: 10.1161/01.res.47.4.501. [DOI] [PubMed] [Google Scholar]
- Wojtczak J. Contractures and increase in internal longitudianl resistance of cow ventricular muscle induced by hypoxia. Circ Res. 1979 Jan;44(1):88–95. doi: 10.1161/01.res.44.1.88. [DOI] [PubMed] [Google Scholar]



