Abstract
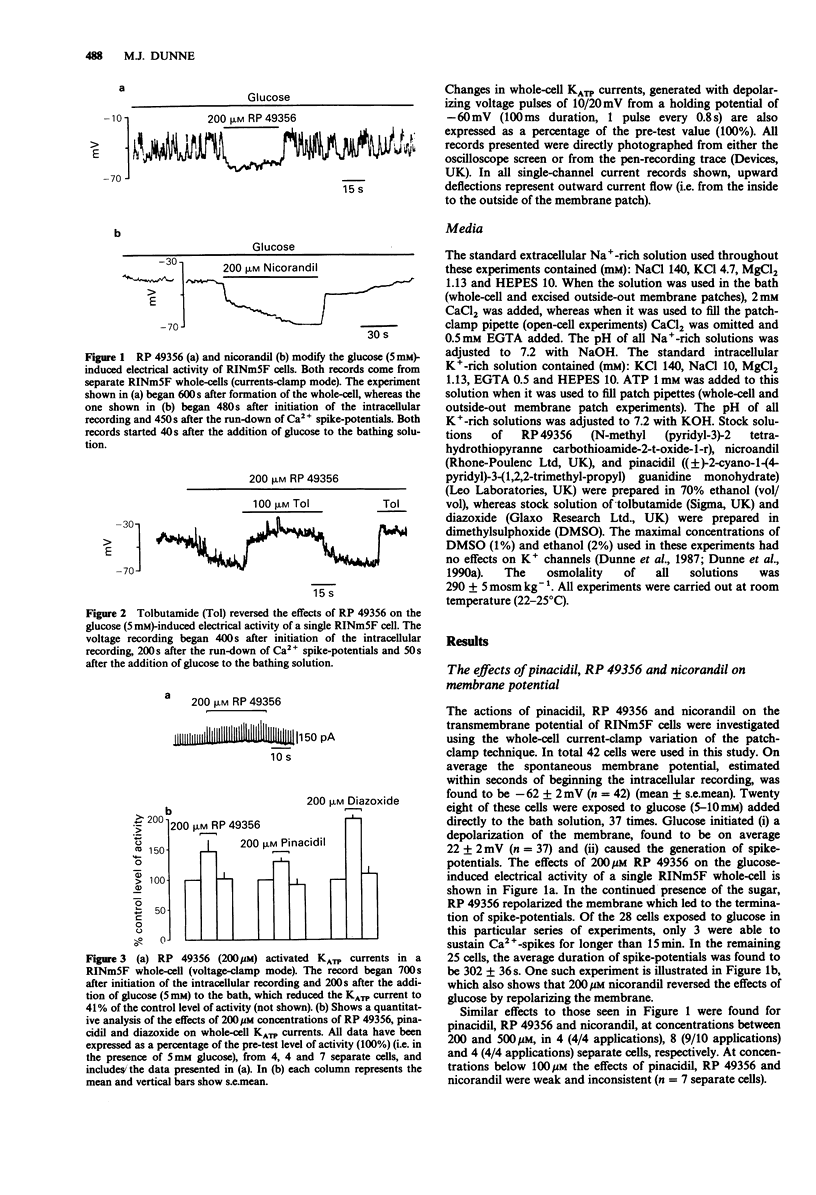
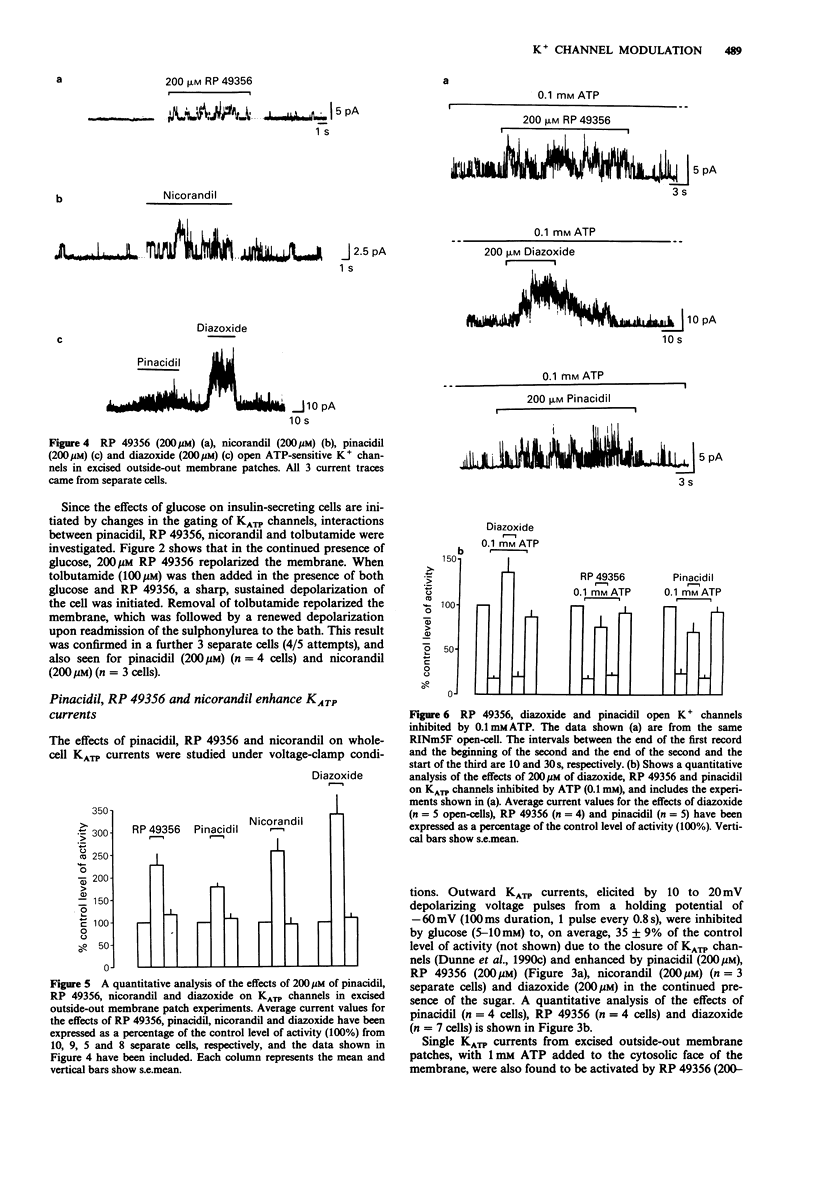
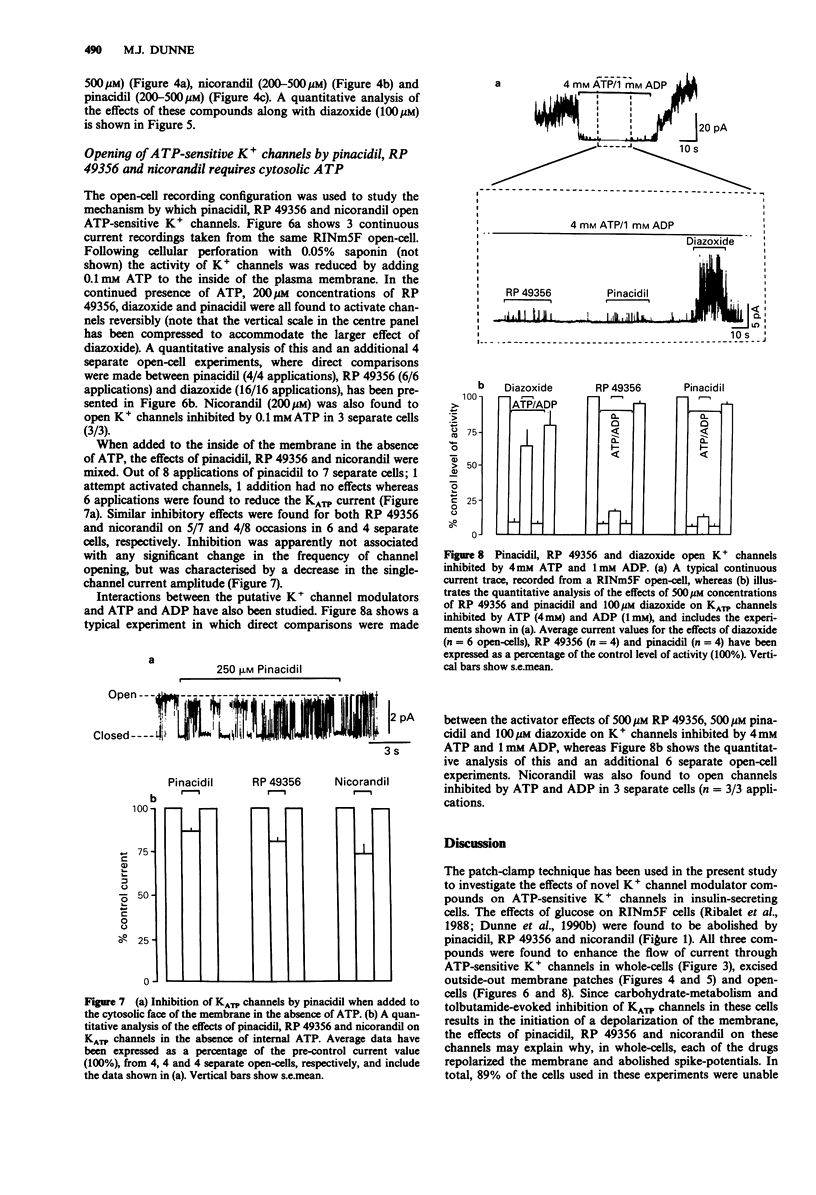
1. The whole-cell patch-clamp technique has been used to investigate the effects of pinacidil, RP 49356 and nicorandil on membrane potential and adenosine 5'-triphosphate (ATP)-sensitive K+ channel currents in the insulin-secreting cell line RINm5F. Interactions between pinacidil, RP 49356, nicorandil, diazoxide and ATP have been studied in excised outside-out membrane patches and open-cells. 2. In RINm5F whole-cells (current-clamp mode) continually exposed to glucose, pinacidil, RP 49356 and nicorandil, at concentrations greater than 100 microM, consistently reversed the effects of the sugar by repolarizing the membrane and terminating voltage-gated Ca2+ spike-potentials. 3. The actions of pinacidil, RP 49356 and nicorandil on membrane potential may be explained by their effects on the opening of ATP-sensitive K+ channels, since all three compounds activated channels in whole-cells (voltage-clamp mode), excised outside-out membrane patches and open-cells, at concentrations greater than 100 microM. Below 100 microM the actions of pinacidil, RP 49356 and nicorandil were weak and inconsistent. 4. The mechanism of channel activation appears to depend on the presence of cytosolic ATP, since in its absence, pinacidil, RP 49356 and nicorandil (greater than 100 microM) had either no effects or inhibited K+ channels. 5. Pinacidil, nicorandil and RP 49356 (200-500 microM) also appeared to open K+ channels inhibited by quasi-physiological concentrations of ATP (4 mM) and ADP (1 mM). However, in comparison to diazoxide their effects were weak.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Moraru E., Hampshire J. On the mechanism of diazoxide-induced hyperglycemia. Diabetes. 1977 Oct;26(10):931–935. doi: 10.2337/diab.26.10.931. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Bray K. M., Newgreen D. T., Small R. C., Southerton J. S., Taylor S. G., Weir S. W., Weston A. H. Evidence that the mechanism of the inhibitory action of pinacidil in rat and guinea-pig smooth muscle differs from that of glyceryl trinitrate. Br J Pharmacol. 1987 Jun;91(2):421–429. doi: 10.1111/j.1476-5381.1987.tb10297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Quast U., Hof R. P., Baumlin Y., Pally C. Similarities in the mechanism of action of two new vasodilator drugs: pinacidil and BRL 34915. J Cardiovasc Pharmacol. 1988 Jan;11(1):90–99. doi: 10.1097/00005344-198801000-00014. [DOI] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Aspinall R. J., Petersen O. H. The effects of cromakalim on ATP-sensitive potassium channels in insulin-secreting cells. Br J Pharmacol. 1990 Jan;99(1):169–175. doi: 10.1111/j.1476-5381.1990.tb14672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Bullett M. J., Li G. D., Wollheim C. B., Petersen O. H. Galanin activates nucleotide-dependent K+ channels in insulin-secreting cells via a pertussis toxin-sensitive G-protein. EMBO J. 1989 Feb;8(2):413–420. doi: 10.1002/j.1460-2075.1989.tb03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Findlay I., Petersen O. H. Effects of pyridine nucleotides on the gating of ATP-sensitive potassium channels in insulin-secreting cells. J Membr Biol. 1988 Jun;102(3):205–216. doi: 10.1007/BF01925714. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Findlay I., Petersen O. H., Wollheim C. B. ATP-sensitive K+ channels in an insulin-secreting cell line are inhibited by D-glyceraldehyde and activated by membrane permeabilization. J Membr Biol. 1986;93(3):271–279. doi: 10.1007/BF01871181. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Illot M. C., Peterson O. H. Interaction of diazoxide, tolbutamide and ATP4- on nucleotide-dependent K+ channels in an insulin-secreting cell line. J Membr Biol. 1987;99(3):215–224. doi: 10.1007/BF01995702. [DOI] [PubMed] [Google Scholar]
- Dunne M. J. Protein phosphorylation is required for diazoxide to open ATP-sensitive potassium channels in insulin (RINm5F) secreting cells. FEBS Lett. 1989 Jul 3;250(2):262–266. doi: 10.1016/0014-5793(89)80734-3. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., West-Jordan J. A., Abraham R. J., Edwards R. H., Petersen O. H. The gating of nucleotide-sensitive K+ channels in insulin-secreting cells can be modulated by changes in the ratio ATP4-/ADP3- and by nonhydrolyzable derivatives of both ATP and ADP. J Membr Biol. 1988 Sep;104(2):165–177. doi: 10.1007/BF01870928. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Charles S., Nenquin M., Mathot F., Tamagawa T. Diazoxide and D600 inhibition of insulin release. Distinct mechanisms explain the specificity for different stimuli. Diabetes. 1982 Sep;31(9):776–783. doi: 10.2337/diab.31.9.776. [DOI] [PubMed] [Google Scholar]
- Hermsmeyer R. K. Pinacidil actions on ion channels in vascular muscle. J Cardiovasc Pharmacol. 1988;12 (Suppl 2):S17–S22. doi: 10.1097/00005344-198812002-00005. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Hales C. N., Ashford M. L. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989 Aug;97(4):1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun P., Devreux V., Hermann M., Herchuelz A. Pinacidil inhibits insulin release by increasing K+ outflow from pancreatic B-cells. Eur J Pharmacol. 1988 Nov 1;156(2):283–286. doi: 10.1016/0014-2999(88)90334-2. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975 Mar;246(2):421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Dunne M. J. Regulation of K+ channels plays a crucial role in the control of insulin secretion. Pflugers Arch. 1989;414 (Suppl 1):S115–S120. doi: 10.1007/BF00582259. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Ribalet B., Eddlestone G. T., Ciani S. Metabolic regulation of the K(ATP) and a maxi-K(V) channel in the insulin-secreting RINm5F cell. J Gen Physiol. 1988 Aug;92(2):219–237. doi: 10.1085/jgp.92.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Thuringer D., Escande D. The potassium channel opener RP 49356 modifies the ATP-sensitivity of K+-ATP channels in cardiac myocytes. Pflugers Arch. 1989;414 (Suppl 1):S175–S175. doi: 10.1007/BF00582290. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Velasco J. M., Petersen J. U., Petersen O. H. Single-channel Ba2+ currents in insulin-secreting cells are activated by glyceraldehyde stimulation. FEBS Lett. 1988 Apr 25;231(2):366–370. doi: 10.1016/0014-5793(88)80851-2. [DOI] [PubMed] [Google Scholar]
- Velasco J. M., Petersen O. H. The effect of a cell-permeable diacylglycerol analogue on single Ca2+ (Ba2+) channel currents in the insulin-secreting cell line RINm5F. Q J Exp Physiol. 1989 May;74(3):367–370. doi: 10.1113/expphysiol.1989.sp003280. [DOI] [PubMed] [Google Scholar]
- Videbaek L. M., Aalkjaer C., Mulvany M. J. Effect of pinacidil on norepinephrine- and potassium-induced contractions and membrane potential in rat and human resistance vessels and in rat aorta. J Cardiovasc Pharmacol. 1988;12 (Suppl 2):S23–S29. doi: 10.1097/00005344-198812002-00006. [DOI] [PubMed] [Google Scholar]
- Wilson C., Coldwell M. C., Howlett D. R., Cooper S. M., Hamilton T. C. Comparative effects of K+ channel blockade on the vasorelaxant activity of cromakalim, pinacidil and nicorandil. Eur J Pharmacol. 1988 Aug 2;152(3):331–339. doi: 10.1016/0014-2999(88)90728-5. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Signal transduction in insulin secretion: comparison between fuel stimuli and receptor agonists. Ann N Y Acad Sci. 1986;488:317–333. doi: 10.1111/j.1749-6632.1986.tb46568.x. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Dunne M. J., Peter-Riesch B., Bruzzone R., Pozzan T., Petersen O. H. Activators of protein kinase C depolarize insulin-secreting cells by closing K+ channels. EMBO J. 1988 Aug;7(8):2443–2449. doi: 10.1002/j.1460-2075.1988.tb03090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


