Abstract
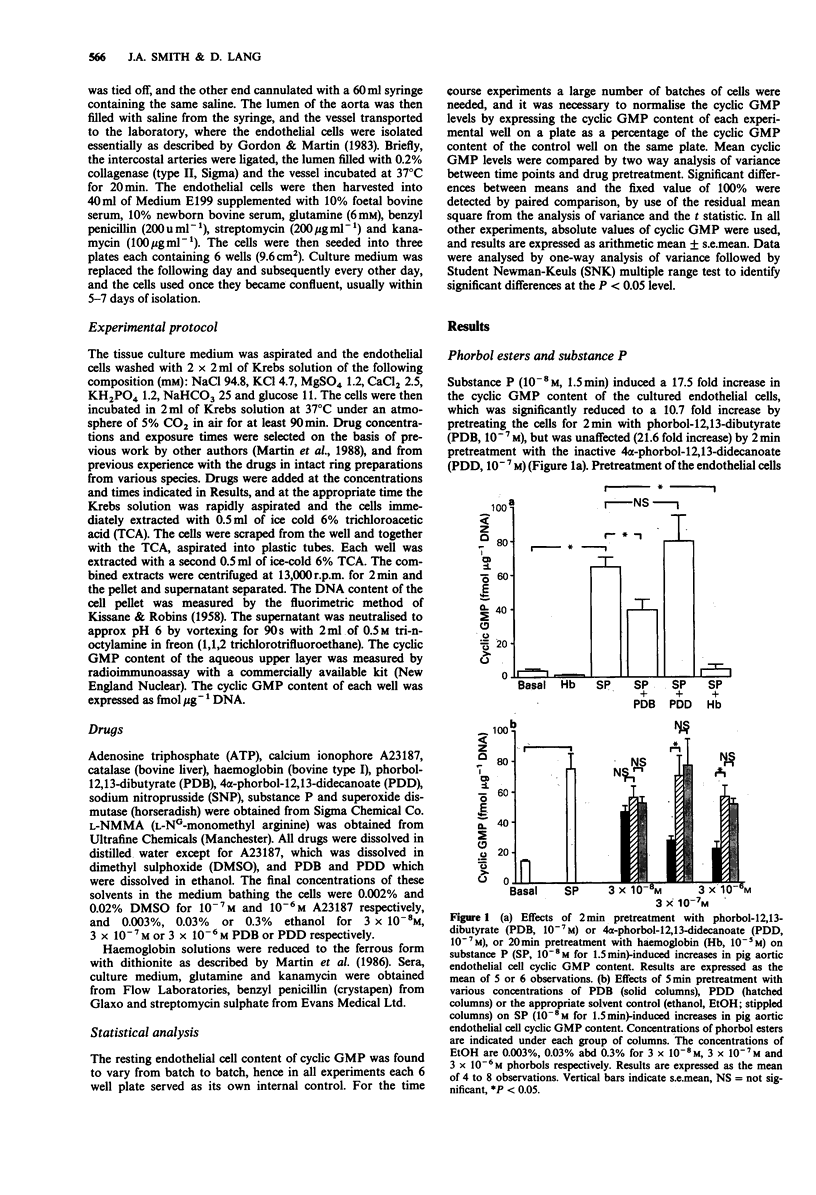
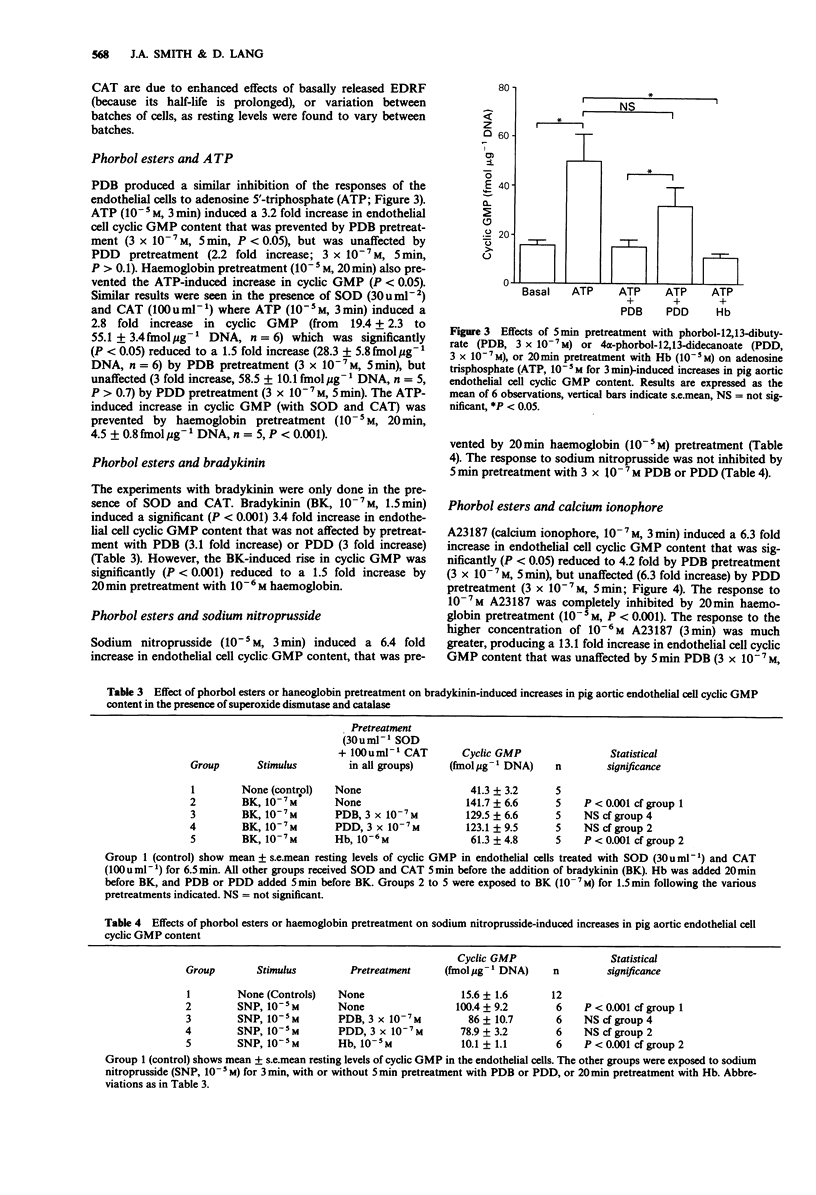
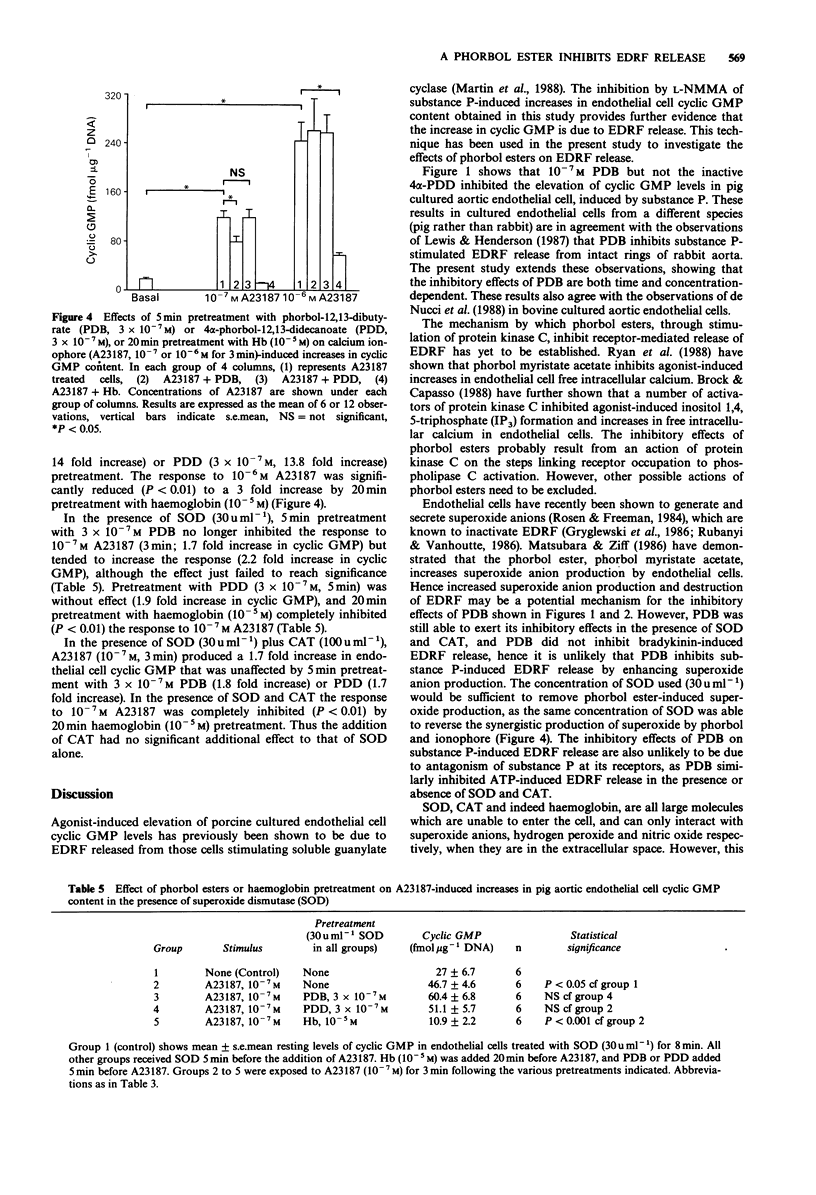
1. Cultured aortic endothelial cells of the pig respond to the endothelium-derived relaxing factor (EDRF) they release with an increase in cyclic GMP content. This response is inhibited by haemoglobin or by L-NG-monomethyl-arginine (L-NMMA), and has been used to investigate the effects of phorbol esters on EDRF release. 2. Pretreatment with phorbol-12,13-dibutyrate (PDB) but not the inactive 4 alpha-phorbol-12,13,-didecanoate (PDD), inhibited increases in cyclic GMP induced by substance P (10(-8) M) in a time and concentration-dependent manner. PDB did not affect basal cyclic GMP levels. 3. PDB (3 x 10(-7) M), but not PDD (3 x 10(-7) M), also inhibited ATP (10(-5) M)-induced increases in cyclic GMP, but did not affect those induced by bradykinin (10(-7) M). 4. Increases in cyclic GMP induced by low (10(-7) M) but not high (10(-6) M) concentrations of the calcium ionophore A23187 were inhibited by PDB (3 x 10(-7) M). This inhibitory effect was due to enhanced destruction of EDRF by superoxide anions rather than inhibition of EDRF release, as the inhibition was abolished in the presence of superoxide dismutase (SOD, 30 mu ml-1) and catalase (CAT, 100 mu ml-1). 5. SOD and CAT did not affect the inhibitory action of PDB on substance P or ATP-induced increases in cyclic GMP. 6. Increases in endothelial cell cyclic GMP content induced by sodium nitroprusside (10(-5) M) were unaffected by PDB pretreatment.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aub D. L., Putney J. W., Jr Properties of receptor-controlled inositol trisphosphate formation in parotid acinar cells. Biochem J. 1985 Jan 1;225(1):263–266. doi: 10.1042/bj2250263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braughler J. M., Mittal C. K., Murad F. Effects of thiols, sugars, and proteins on nitric oxide activation of guanylate cyclase. J Biol Chem. 1979 Dec 25;254(24):12450–12454. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Evans H. G., Smith J. A., Lewis M. J. Release of endothelium-derived relaxing factor is inhibited by 8-bromo-cyclic guanosine monophosphate. J Cardiovasc Pharmacol. 1988 Dec;12(6):672–677. doi: 10.1097/00005344-198812000-00008. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or non-heme iron. Eur J Pharmacol. 1987 Oct 27;142(3):465–469. doi: 10.1016/0014-2999(87)90090-2. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A., Shimokawa H., Vanhoutte P. M. Pertussis toxin inhibits endothelium-dependent relaxations to certain agonists in porcine coronary arteries. J Physiol. 1989 Jan;408:549–560. doi: 10.1113/jphysiol.1989.sp017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hogan J. C., Smith J. A., Richards A. C., Lewis M. J. Atrial natriuretic peptide inhibits the release of endothelium-derived relaxing factor from blood vessels of the rabbit. Eur J Pharmacol. 1989 Jun 8;165(1):129–134. doi: 10.1016/0014-2999(89)90778-4. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Pessin J. E., Ruoho A. E., Johnson G. L. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman D. C., Murad F. Comparison of binding and cyclic GMP accumulation by atrial natriuretic peptides in endothelial cells. Biochim Biophys Acta. 1986 Jan 23;885(1):74–79. doi: 10.1016/0167-4889(86)90040-6. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Collins P., Lang D. Endothelium-derived relaxing factor, calcium and inositol phosphates. Biochem Soc Trans. 1988 Aug;16(4):486–488. doi: 10.1042/bst0160486. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Henderson A. H. A phorbol ester inhibits the release of endothelium-derived relaxing factor. Eur J Pharmacol. 1987 Jun 4;137(2-3):167–171. doi: 10.1016/0014-2999(87)90218-4. [DOI] [PubMed] [Google Scholar]
- Martin W., Smith J. A., White D. G. The mechanisms by which haemoglobin inhibits the relaxation of rabbit aorta induced by nitrovasodilators, nitric oxide, or bovine retractor penis inhibitory factor. Br J Pharmacol. 1986 Nov;89(3):563–571. doi: 10.1111/j.1476-5381.1986.tb11157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Avdonin P. V., Posin E. Y., Popov E. G., Danilov S. M., Tkachuk V. A. Influence of vasoactive agents on cytoplasmic free calcium in vascular endothelial cells. J Appl Physiol (1985) 1988 Nov;65(5):2221–2227. doi: 10.1152/jappl.1988.65.5.2221. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gárdos G. The use of ionophores of rapid loading of human red cells with radioactive cations for cation-pump studies. J Membr Biol. 1976 May;26(4):357–370. doi: 10.1007/BF01868883. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Nambi P., Peters J. R., Lefkowitz R. J. Phorbol diesters promote beta-adrenergic receptor phosphorylation and adenylate cyclase desensitization in duck erythrocytes. Biochem Biophys Res Commun. 1984 Jun 29;121(3):973–979. doi: 10.1016/0006-291x(84)90772-1. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanonaka K., Matsumoto M., Minematsu R., Miyake K., Murai R., Takeo S. Beneficial effect of amosulalol and phentolamine on post-hypoxic recovery of contractile force and energy metabolism in rabbit hearts. Br J Pharmacol. 1989 Jun;97(2):513–523. doi: 10.1111/j.1476-5381.1989.tb11980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., Downes C. P. Substance P induced hydrolysis of inositol phospholipids in guinea-pig ileum and rat hypothalamus. Eur J Pharmacol. 1983 Sep 30;93(3-4):245–253. doi: 10.1016/0014-2999(83)90144-9. [DOI] [PubMed] [Google Scholar]
- Weinheimer G., Wagner B., Osswald H. Interference of phorbolesters with endothelium-dependent vascular smooth muscle relaxation. Eur J Pharmacol. 1986 Nov 4;130(3):319–322. doi: 10.1016/0014-2999(86)90285-2. [DOI] [PubMed] [Google Scholar]
- White A. A., Crawford K. M., Patt C. S., Lad P. J. Activation of soluble guanylate cyclase from rat lung by incubation or by hydrogen peroxide. J Biol Chem. 1976 Dec 10;251(23):7304–7312. [PubMed] [Google Scholar]
- Yousufzai S. Y., Akhtar R. A., Abdel-Latif A. A. Effects of substance P on inositol triphosphate accumulation, on contractile responses and on arachidonic acid release and prostaglandin biosynthesis in rabbit iris sphincter muscle. Exp Eye Res. 1986 Aug;43(2):215–226. doi: 10.1016/s0014-4835(86)80089-6. [DOI] [PubMed] [Google Scholar]
- Zwiller J., Revel M. O., Malviya A. N. Protein kinase C catalyzes phosphorylation of guanylate cyclase in vitro. J Biol Chem. 1985 Feb 10;260(3):1350–1353. [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]


