Abstract
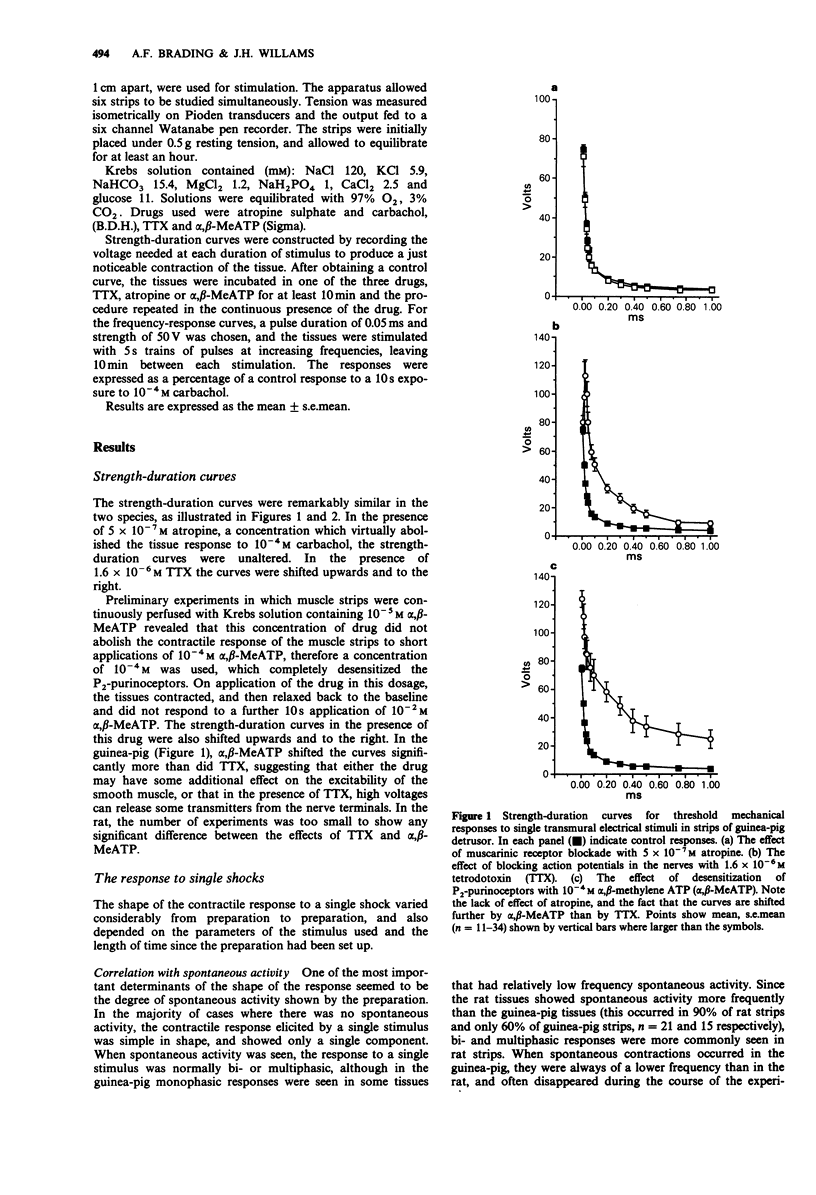
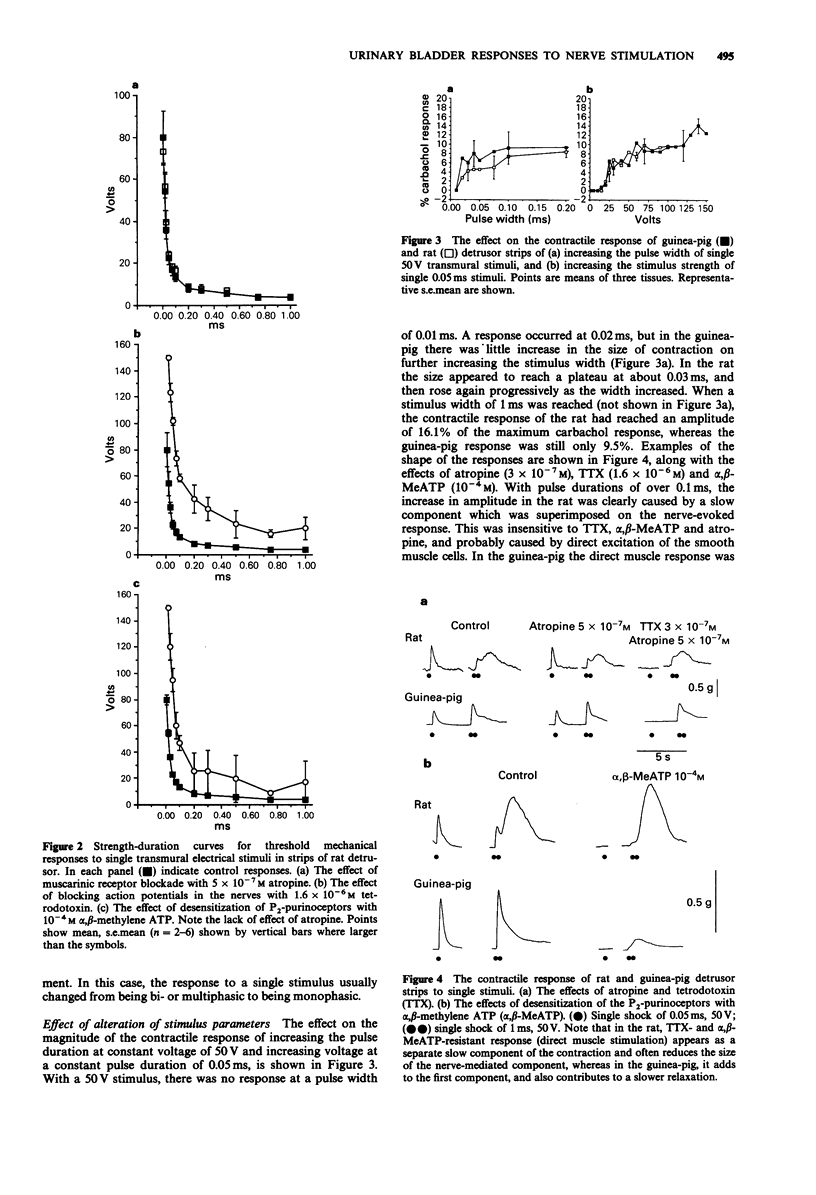
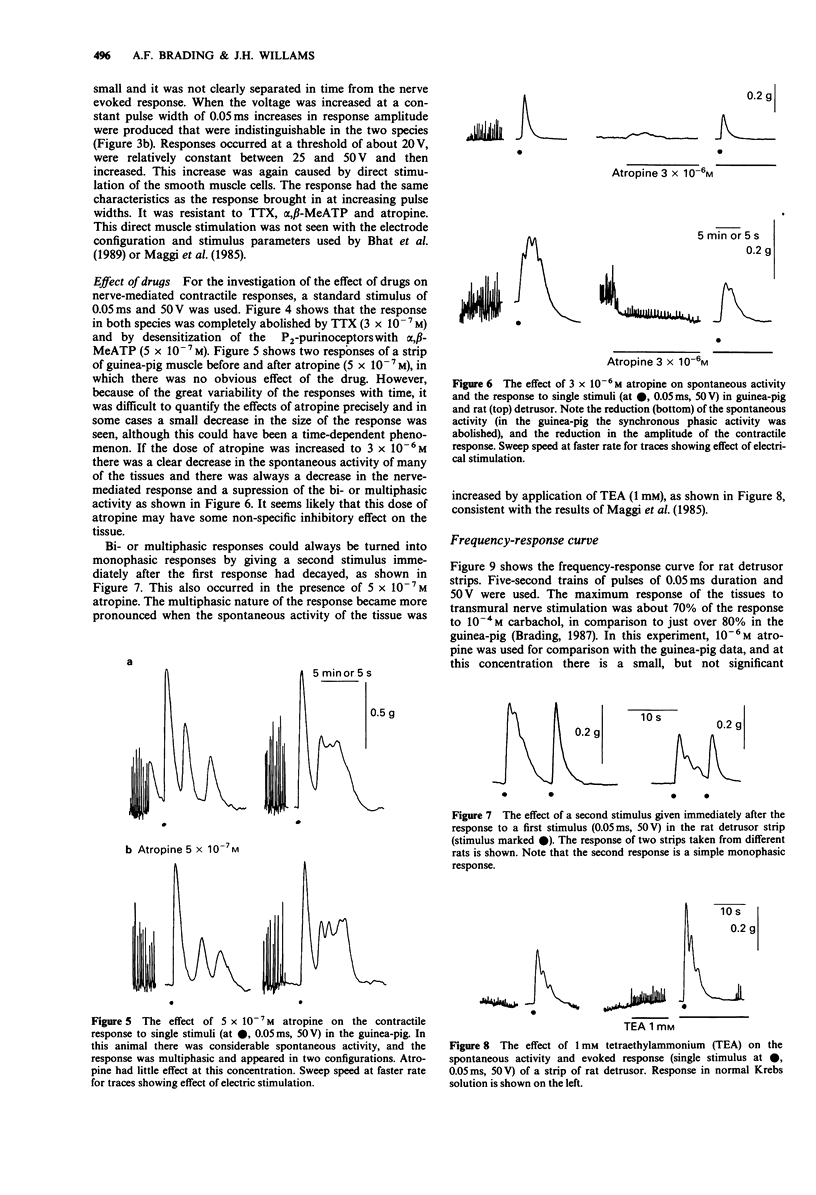
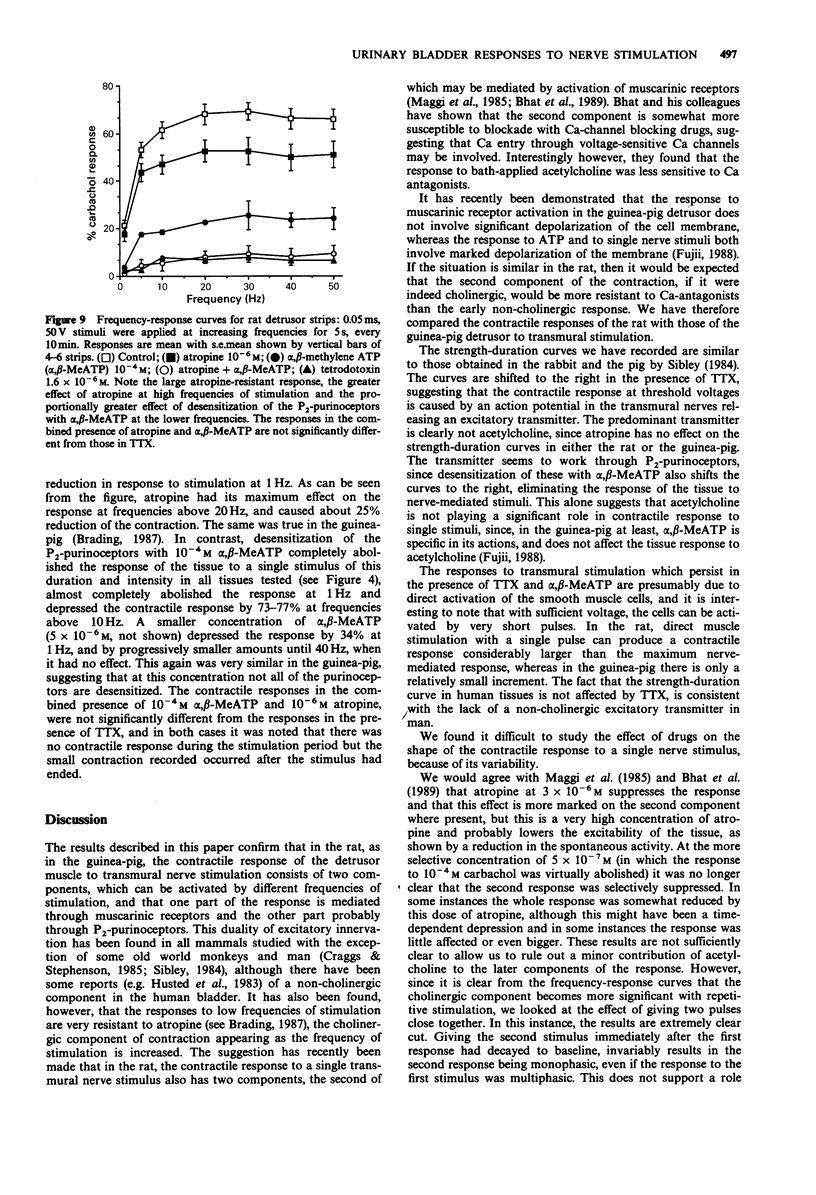
1. Strength-duration curves for threshold mechanical responses to single transmural stimuli were identical for rat and guinea-pig detrusor. In both species atropine had no effect on the curves, but the curves were shifted to the right by nerve blockade with tetrodotoxin (TTX), and by blockade of P2-purinoceptors with alpha,beta-methylene ATP (alpha,beta-MeATP). 2. With short duration pulses of 50 V and less, the responses were nerve-mediated. Increase in either the strength or duration of the stimulus caused direct muscle stimulation, resistant to blockade with atropine, TTX and alpha-beta-MeATP. 3. The shape of the contractile response to a single nerve stimulus varied from tissue to tissue. The responses could be mono-, bi-, or multiphasic. Bi- or multiphasic responses were normally seen in tissues which were spontaneously active. The multiphasic nature of the response was enhanced by factors which increased the excitability of the cells and was reduced by factors which decreased the excitability. 4. The frequency-response curves in the rat are similar to those previously obtained in the guinea-pig. Atropine suppresses the high frequency response by 25%, with little effect at low frequencies, whereas desensitization of P2-purinoceptors with alpha,beta-MeATP suppresses the responses maximally at low frequencies but still by 75% at high frequencies. A combination of both drugs eliminates the nerve-mediated responses. 5. It is concluded that the response to a single nerve stimulus is mediated by a non-cholinergic transmitter, through activation of P2-purinoceptors. The possibility that simultaneous release of acetylcholine can modify the excitability of cells and thus the configuration of the response to a single stimulus is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat M. B., Mishra S. K., Raviprakash V. Differential susceptibility of cholinergic and noncholinergic neurogenic responses to calcium channel blockers and low Ca2+ medium in rat urinary bladder. Br J Pharmacol. 1989 Apr;96(4):837–842. doi: 10.1111/j.1476-5381.1989.tb11892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Kasakov L., Wong H. K. Direct evidence for ATP release from non-adrenergic, non-cholinergic ("purinergic") nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol. 1978 May 15;49(2):145–149. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- CLARK B. B., URSILLO R. C. The action of atropine on the urinary bladder of the dog and on the isolated nerve-bladder strip preparation of the rabbit. J Pharmacol Exp Ther. 1956 Nov;118(3):338–347. [PubMed] [Google Scholar]
- Craggs M. D., Stephenson J. D. Bladder electromyograms and function in monkeys after atropine. Br J Urol. 1985 Jun;57(3):341–345. doi: 10.1111/j.1464-410x.1985.tb06358.x. [DOI] [PubMed] [Google Scholar]
- Creed K. E., Ishikawa S., Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983 May;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol. 1988 Oct;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by alpha,beta-methylene ATP. Eur J Pharmacol. 1985 Aug 15;114(2):239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Husted S., Sjögren C., Andersson K. E. Direct effects of adenosine and adenine nucleotides on isolated human urinary bladder and their influence on electrically induced contractions. J Urol. 1983 Aug;130(2):392–398. doi: 10.1016/s0022-5347(17)51175-1. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895 Dec 30;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Meli A. Pharmacological evidence for the existence of two components in the twitch response to field stimulation of detrusor strips from the rat urinary bladder. J Auton Pharmacol. 1985 Sep;5(3):221–229. doi: 10.1111/j.1474-8673.1985.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Sibley G. N. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984 Sep;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]


