Abstract
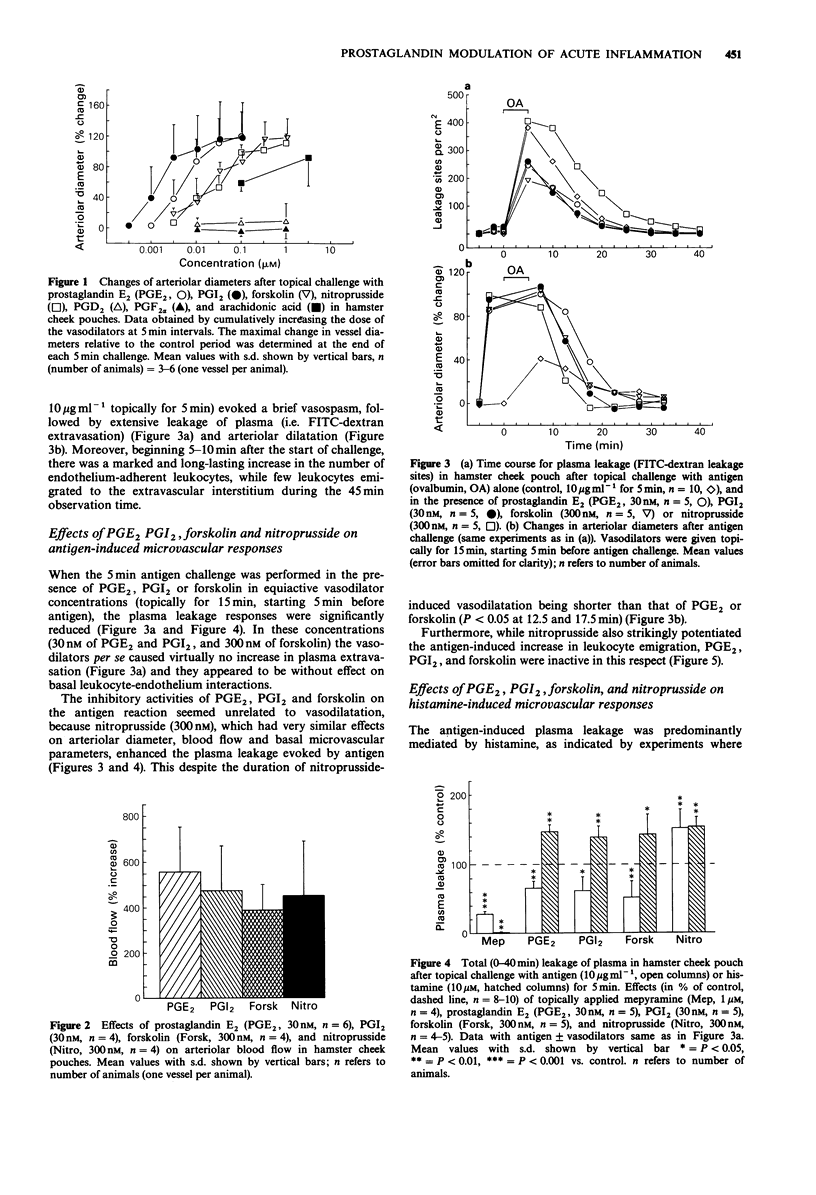
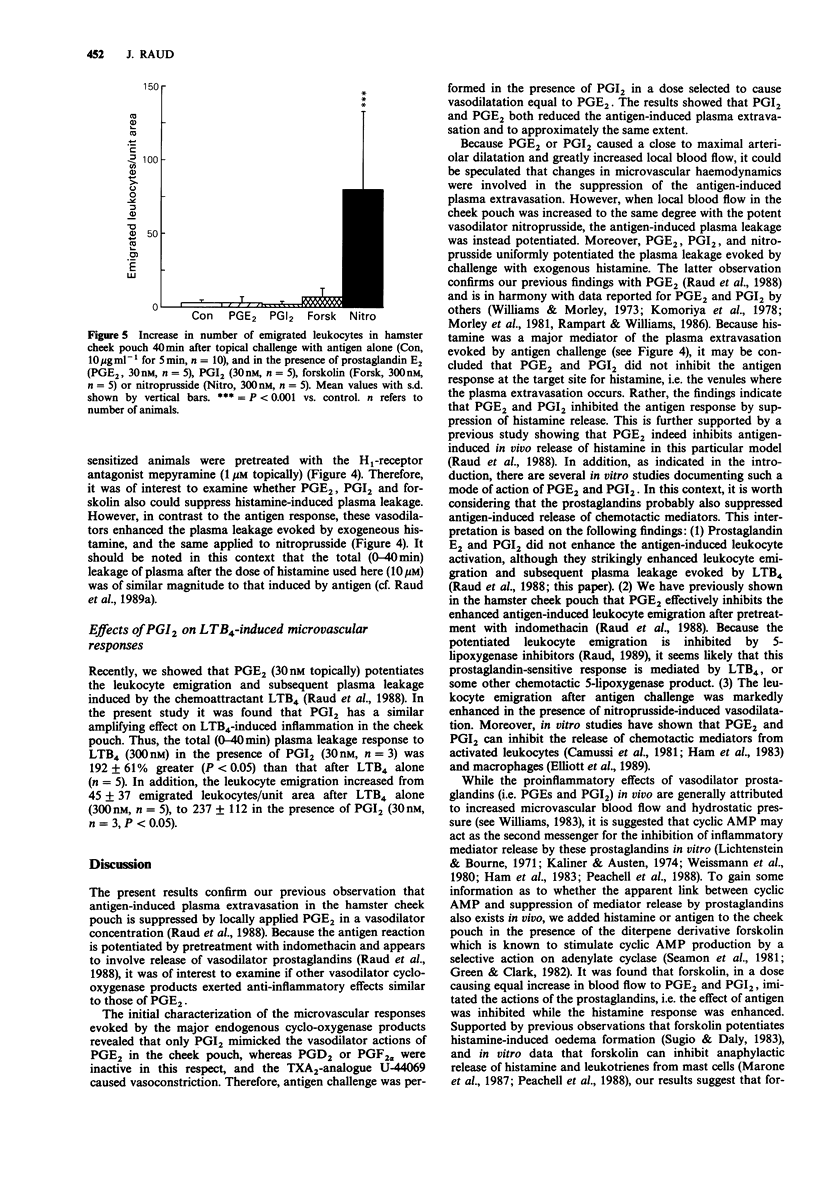
1. Intravital microscopy of the hamster cheek pouch was used to examine the influence of vasodilator prostanoids (prostaglandin E2 (PGE2), PGI2), forskolin, and nitroprusside on the microvascular changes during acute inflammation induced by antigen or histamine. The results extend our previous finding that PGE2 modulates allergic inflammation and histamine release in the cheek pouch model. 2. The microvascular actions of arachidonic acid and different cyclo-oxygenase products (PGE2, PGD2, PGI2, PGF2 alpha, and the thromboxane A2 (TXA2)-analogue U-44069) were first compared with respect to their effects on arteriolar tone. Of the prostaglandins, only PGE2 and PGI2 were potent vasodilators and markedly increased local blood flow. Nitroprusside and forskolin also caused vasodilatation and increased blood flow, but were somewhat less potent than PGE2 and PGI2. 3. Topically applied PGE2 and PGI2 in vasodilator concentrations suppressed the antigen-induced plasma leakage. On the other hand, although the antigen response was predominantly mediated by histamine, both prostaglandins enhanced the plasma leakage evoked by exogenous histamine. 4. In contrast, the vasodilator nitroprusside, in a dose causing an increase in blood flow equal to that of PGE2 and PGI2, potentiated both the histamine-induced plasma leakage, as well as the plasma and leukocyte extravasation after antigen challenge, indicating that the anti-inflammatory actions of the prostaglandins were unrelated to their vasodilator properties per se.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk J., Hedqvist P., Arfors K. E. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation. 1982 Jun;6(2):189–200. doi: 10.1007/BF00916243. [DOI] [PubMed] [Google Scholar]
- Bray M. A., Cunningham F. M., Ford-Hutchinson A. W., Smith M. J. Leukotriene B4: a mediator of vascular permeability. Br J Pharmacol. 1981 Mar;72(3):483–486. doi: 10.1111/j.1476-5381.1981.tb11000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Segoloni G., Chiara Deregibus M., Bussolino F. Neutropenia induced by platelet-activating factor (PAF-acether) released from neutrophils: the inhibitory effect of prostacyclin (PGI2). Agents Actions. 1981 Dec;11(6-7):550–553. doi: 10.1007/BF01978735. [DOI] [PubMed] [Google Scholar]
- Crunkhorn P., Willis A. L. Cutaneous reactions to intradermal prostaglandins. Br J Pharmacol. 1971 Jan;41(1):49–56. doi: 10.1111/j.1476-5381.1971.tb09934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant B., Krüger P. G. Histamine release from isolated rat peritoneal mast cells induced by adenosine-5'-triphosphate. Acta Physiol Scand. 1967 Dec;71(4):291–302. doi: 10.1111/j.1748-1716.1967.tb03736.x. [DOI] [PubMed] [Google Scholar]
- Duling B. R. The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc Res. 1973 May;5(3):423–429. doi: 10.1016/0026-2862(73)90059-9. [DOI] [PubMed] [Google Scholar]
- Elliott G. R., Lauwen A. P., Bonta I. L. Prostaglandin E2 inhibits and indomethacin and aspirin enhance, A23187-stimulated leukotriene B4 synthesis by rat peritoneal macrophages. Br J Pharmacol. 1989 Feb;96(2):265–270. doi: 10.1111/j.1476-5381.1989.tb11812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer D. M., Jose P. J., Piper P. J., Tippins J. R. Modulation of slow-reacting substance of anaphylaxis and histamine release by prostacyclin and thromboxanes [proceedings]. J Physiol. 1978 Aug;281:42P–42P. [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Green D. A., Clark R. B. Direct evidence for the role of the coupling proteins in forskolin activation of adenylate cyclase. J Cyclic Nucleotide Res. 1982;8(5):337–346. [PubMed] [Google Scholar]
- Ham E. A., Soderman D. D., Zanetti M. E., Dougherty H. W., McCauley E., Kuehl F. A., Jr Inhibition by prostaglandins of leukotriene B4 release from activated neutrophils. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4349–4353. doi: 10.1073/pnas.80.14.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs G. A., Eakins K. E., Mugridge K. G., Moncada S., Vane J. R. The effects of non-steroid anti-inflammatory drugs on leukocyte migration in carrageenin-induced inflammation. Eur J Pharmacol. 1980 Aug 22;66(1):81–86. doi: 10.1016/0014-2999(80)90297-6. [DOI] [PubMed] [Google Scholar]
- Hitchcock M. Effect of inhibitors of prostaglandin synthesis and prostaglandins E2 and F2alpha on the immunologic release of mediators of inflammation from actively sensitized guinea-pig lung. J Pharmacol Exp Ther. 1978 Nov;207(2):630–640. [PubMed] [Google Scholar]
- Kaliner M., Austen K. F. Cyclic AMP, ATP, and reversed anaphylactic histamine release from rat mast cells. J Immunol. 1974 Feb;112(2):664–674. [PubMed] [Google Scholar]
- Kiernan J. A. Effects of known and suspected neurotransmitter substances and of some nucleotides on isolated mast cells. Experientia. 1972 Jun 15;28(6):653–655. doi: 10.1007/BF01944958. [DOI] [PubMed] [Google Scholar]
- Komoriya K., Ohmori H., Azuma A., Kurozumi S., Hashimoto Y., Nicolaou K. C., Barnette W. E., Magolda R. L. Prostaglandin I2 as a potentiator of acute inflammation in rats. Prostaglandins. 1978 Apr;15(4):557–564. doi: 10.1016/0090-6980(78)90052-7. [DOI] [PubMed] [Google Scholar]
- Loeffler L. J., Lovenberg W., Sjoerdsma A. Effects of dibutyryl-3',5'-cyclic adenosine monophosphage, phosphodiesterase inhibitors and prostaglandin E1 on compound 48-80-induced histamine release from rat peritoneal mast cells in vitro. Biochem Pharmacol. 1971 Sep;20(9):2287–2297. doi: 10.1016/0006-2952(71)90228-0. [DOI] [PubMed] [Google Scholar]
- Lundberg C., Gerdin B. The inflammatory reaction in an experimental model of open wounds in the rat. The effect of arachidonic acid metabolites. Eur J Pharmacol. 1984 Jan 27;97(3-4):229–238. doi: 10.1016/0014-2999(84)90454-0. [DOI] [PubMed] [Google Scholar]
- Marone G., Columbo M., Triggiani M., Cirillo R., Genovese A., Formisano S. Inhibition of IgE-mediated release of histamine and peptide leukotriene from human basophils and mast cells by forskolin. Biochem Pharmacol. 1987 Jan 1;36(1):13–20. doi: 10.1016/0006-2952(87)90377-7. [DOI] [PubMed] [Google Scholar]
- Morley J., Page C., Paul W. Leucotrienes, SRS-A and the vascular manifestations of PCA. Agents Actions. 1981 Dec;11(6-7):585–587. doi: 10.1007/BF01978752. [DOI] [PubMed] [Google Scholar]
- Peachell P. T., MacGlashan D. W., Jr, Lichtenstein L. M., Schleimer R. P. Regulation of human basophil and lung mast cell function by cyclic adenosine monophosphate. J Immunol. 1988 Jan 15;140(2):571–579. [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Polymorphonuclear leukocyte-dependent plasma leakage in the rabbit skin is enhanced or inhibited by prostacyclin, depending on the route of administration. Am J Pathol. 1986 Jul;124(1):66–73. [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Raud J., Dahlén S. E., Smedegård G., Hedqvist P. An intravital microscopic model for mast cell-dependent inflammation in the hamster cheek pouch. Acta Physiol Scand. 1989 Feb;135(2):95–105. doi: 10.1111/j.1748-1716.1989.tb08556.x. [DOI] [PubMed] [Google Scholar]
- Raud J., Dahlén S. E., Sydbom A., Lindbom L., Hedqvist P. Enhancement of acute allergic inflammation by indomethacin is reversed by prostaglandin E2: apparent correlation with in vivo modulation of mediator release. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2315–2319. doi: 10.1073/pnas.85.7.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raud J. Intravital microscopic studies on acute mast cell-dependent inflammation. Acta Physiol Scand Suppl. 1989;578:1–58. [PubMed] [Google Scholar]
- Raud J., Lindbom L., Dahlèn S. E., Hedqvist P. Periarteriolar localization of mast cells promotes oriented interstitial migration of leukocytes in the hamster cheek pouch. Am J Pathol. 1989 Jan;134(1):161–169. [PMC free article] [PubMed] [Google Scholar]
- Raud J., Sydbom A., Dahlén S. E., Hedqvist P. Prostaglandin E2 prevents diclofenac-induced enhancement of histamine release and inflammation evoked by in vivo challenge with compound 48/80 in the hamster cheek pouch. Agents Actions. 1989 Aug;28(1-2):108–114. doi: 10.1007/BF02022990. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Sugio K., Daly J. W. Effect of forskolin on alterations of vascular permeability induced with bradykinin, prostaglandin E1, adenosine, histamine and carrageenin in rats. Life Sci. 1983 Jul 4;33(1):65–73. doi: 10.1016/0024-3205(83)90712-9. [DOI] [PubMed] [Google Scholar]
- Svensjö E., Arfors K. E., Arturson G., Rutili G. The hamster cheek pouch preparation as a model for studies of macromolecular permeability of the microvasculature. Ups J Med Sci. 1978;83(1):71–79. doi: 10.3109/03009737809179115. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wayland H., Johnson P. C. Erythrocyte velocity measurement in microvessels by a two-slit photometric method. J Appl Physiol. 1967 Feb;22(2):333–337. doi: 10.1152/jappl.1967.22.2.333. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. Prostaglandins and inflammation: receptor/cyclase coupling as an explanation of why PGEs and PGI2 inhibit functions of inflammatory cells. Adv Prostaglandin Thromboxane Res. 1980;8:1637–1653. [PubMed] [Google Scholar]
- Williams T. J., Hellewell P. G., Jose P. J. Inflammatory mechanisms in the Arthus reaction. Agents Actions. 1986 Oct;19(1-2):66–72. doi: 10.1007/BF01977260. [DOI] [PubMed] [Google Scholar]
- Williams T. J. Interactions between prostaglandins, leukotrienes and other mediators of inflammation. Br Med Bull. 1983 Jul;39(3):239–242. doi: 10.1093/oxfordjournals.bmb.a071826. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]


