Abstract
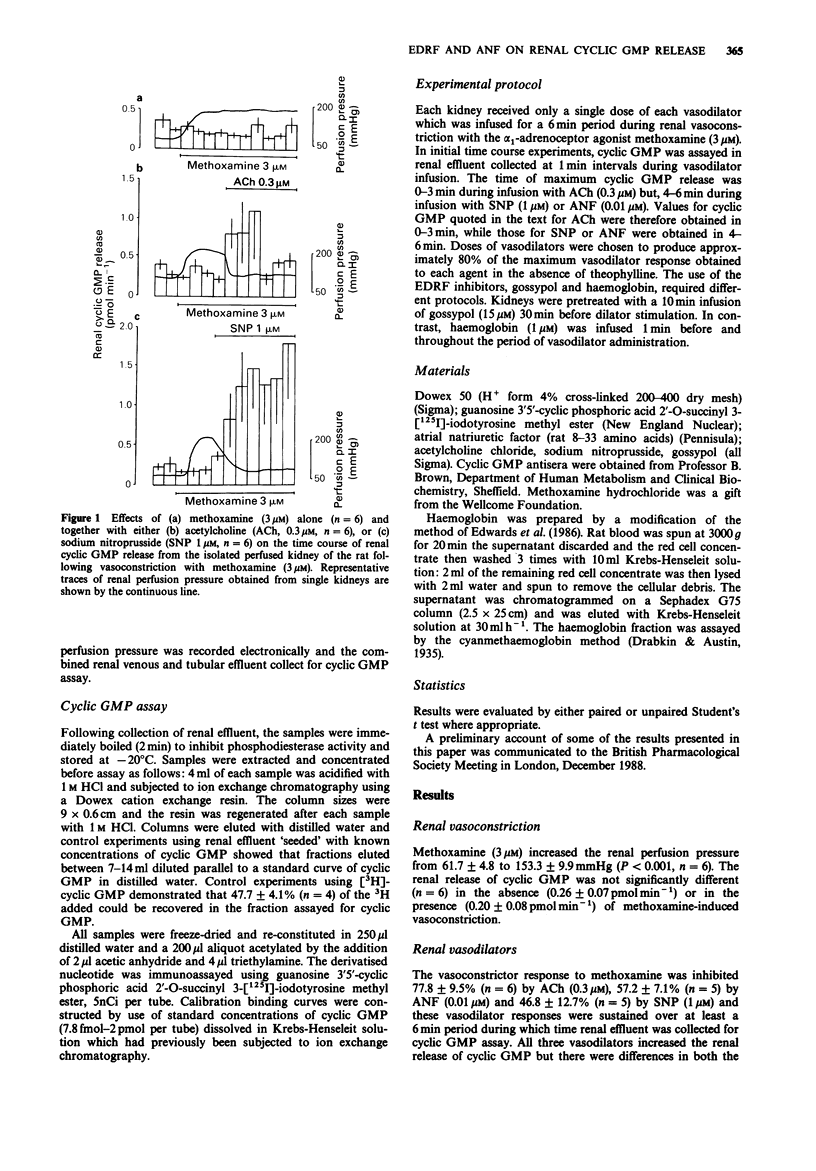
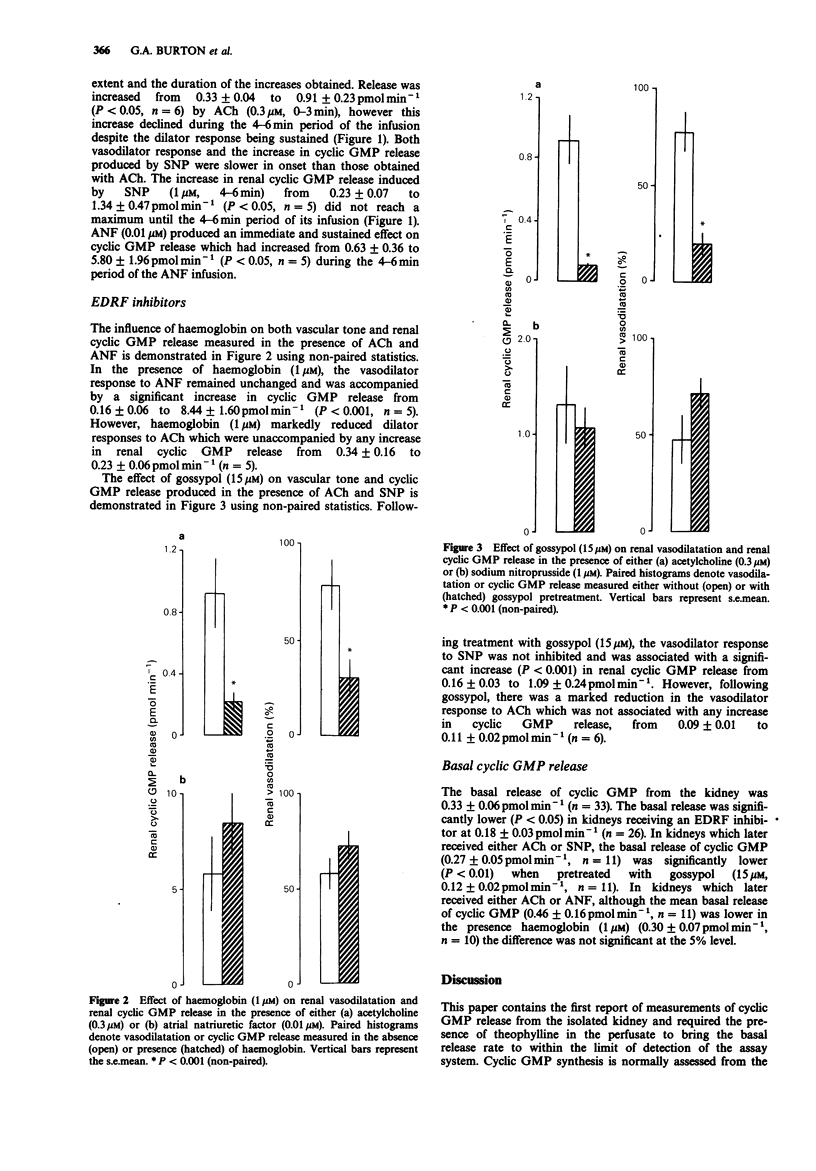
1. Guanosine 3':5'-cyclic monophosphate (cyclic GMP) release and vascular tone was measured in the isolated kidney of the rat perfused at constant flow with Krebs-Henseleit solution. The effects of 3 vasodilators, acetylcholine (ACh), atrial natriuretic factor (ANF) and sodium nitroprusside (SNP) on the renal release of cyclic GMP and vascular tone were examined. The ability of the endothelial-derived relaxing factor (EDRF) inhibitors, haemoglobin and gossypol, to modify vasodilatation and vasodilator-induced changes in cyclic GMP releases from the kidney was also investigated. 2. Renal cyclic GMP release was elevated 8 fold by ANF (0.01 microM), 5 fold by SNP (1 microM) and 3 fold by ACh (0.3 microM). 3. For ACh, both the increase in renal cyclic GMP release and the vasodilatation were reduced by the EDRF inhibitors, haemoglobin (1 microM) and gossypol (15 microM). For SNP, neither the increase in renal cyclic GMP release nor vasodilatation were inhibited by gossypol (15 microM). 4. For ANF, neither the increase in cyclic GMP release from the kidney nor its vasodilator activity were affected by haemoglobin (1 microM). 5. EDRF inhibitors reduced the basal release of cyclic GMP from 0.32 +/- 0.06 pmol min-1 to 0.18 +/- 0.03 pmol min-1, gossypol being more effective than haemoglobin. 6. The results are consistent with the ability of ACh to induce EDRF-mediated vasodilatation in the isolated perfused kidney of the rat. Basal EDRF release appears to contribute approximately 50% to the basal release of cyclic GMP from this preparation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alheid U., Dudel C., Förstermann U. Selective inhibition by gossypol of endothelium-dependent relaxations augments relaxations to glyceryl trinitrate in rabbit coeliac artery. Br J Pharmacol. 1987 Sep;92(1):237–240. doi: 10.1111/j.1476-5381.1987.tb11317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj R., Moore P. K. Endothelium-derived relaxing factor and the effects of acetylcholine and histamine on resistance blood vessels. Br J Pharmacol. 1988 Nov;95(3):835–843. doi: 10.1111/j.1476-5381.1988.tb11712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock G. R., Taylor S. G., Weston A. H. Influence of the vascular endothelium on agonist-induced contractions and relaxations in rat aorta. Br J Pharmacol. 1986 Dec;89(4):819–830. doi: 10.1111/j.1476-5381.1986.tb11187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. H., Griffith T. M., Ryley H. C., Henderson A. H. Haptoglobin-haemoglobin complex in human plasma inhibits endothelium dependent relaxation: evidence that endothelium derived relaxing factor acts as a local autocoid. Cardiovasc Res. 1986 Aug;20(8):549–556. doi: 10.1093/cvr/20.8.549. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957 May 23;136(3):507–524. doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. Endothelium-derived relaxing factor (EDRF) and resistance vessels in an intact vascular bed: a microangiographic study of the rabbit isolated ear. Br J Pharmacol. 1988 Mar;93(3):654–662. doi: 10.1111/j.1476-5381.1988.tb10323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter C. A., Lemke S. M. Comparison of endothelium-dependent relaxation in bovine intrapulmonary artery and vein by acetylcholine and A23187. J Pharmacol Exp Ther. 1986 Sep;238(3):1055–1062. [PubMed] [Google Scholar]
- Linz W., Albus U., Wiemer G., Schölkens B. A., König W. Atriopeptin III induces endothelium-independent relaxation and increases cGMP levels in rabbit aorta. Klin Wochenschr. 1986;64 (Suppl 6):27–30. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pathak H. K., Corder C. N., Suman N. Gossypol, ouabain and vanadate on the isolated perfused rat kidney and Na, K-ATPase. Zhongguo Yao Li Xue Bao. 1984 Mar;5(1):52–57. [PubMed] [Google Scholar]
- Randall M. D., Hiley C. R. Effect of phenobarbitone pretreatment upon endothelium-dependent relaxation to acetylcholine in rat superior mesenteric arterial bed. Br J Pharmacol. 1988 Jul;94(3):977–983. doi: 10.1111/j.1476-5381.1988.tb11612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Schenk D. B., Johnson L. K., Schwartz K., Sista H., Scarborough R. M., Lewicki J. A. Distinct atrial natriuretic factor receptor sites on cultured bovine aortic smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1985 Mar 15;127(2):433–442. doi: 10.1016/s0006-291x(85)80179-0. [DOI] [PubMed] [Google Scholar]
- Takeda S., Kusano E., Murayama N., Asano Y., Hosoda S., Sokabe H., Kawashima H. Atrial natriuretic peptide elevates cGMP contents in glomeruli and in distal tubules of rat kidney. Biochem Biophys Res Commun. 1986 May 14;136(3):947–954. doi: 10.1016/0006-291x(86)90424-9. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Southerton J. S., Weston A. H., Baker J. R. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol. 1988 Jul;94(3):853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M. J., Romero J. C., Vanhoutte P. M. Endothelium-derived relaxing factor inhibits renin release. Eur J Pharmacol. 1988 May 10;149(3):401–402. doi: 10.1016/0014-2999(88)90679-6. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Winquist R. J., Faison E. P., Waldman S. A., Schwartz K., Murad F., Rapoport R. M. Atrial natriuretic factor elicits an endothelium-independent relaxation and activates particulate guanylate cyclase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7661–7664. doi: 10.1073/pnas.81.23.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]


