Abstract
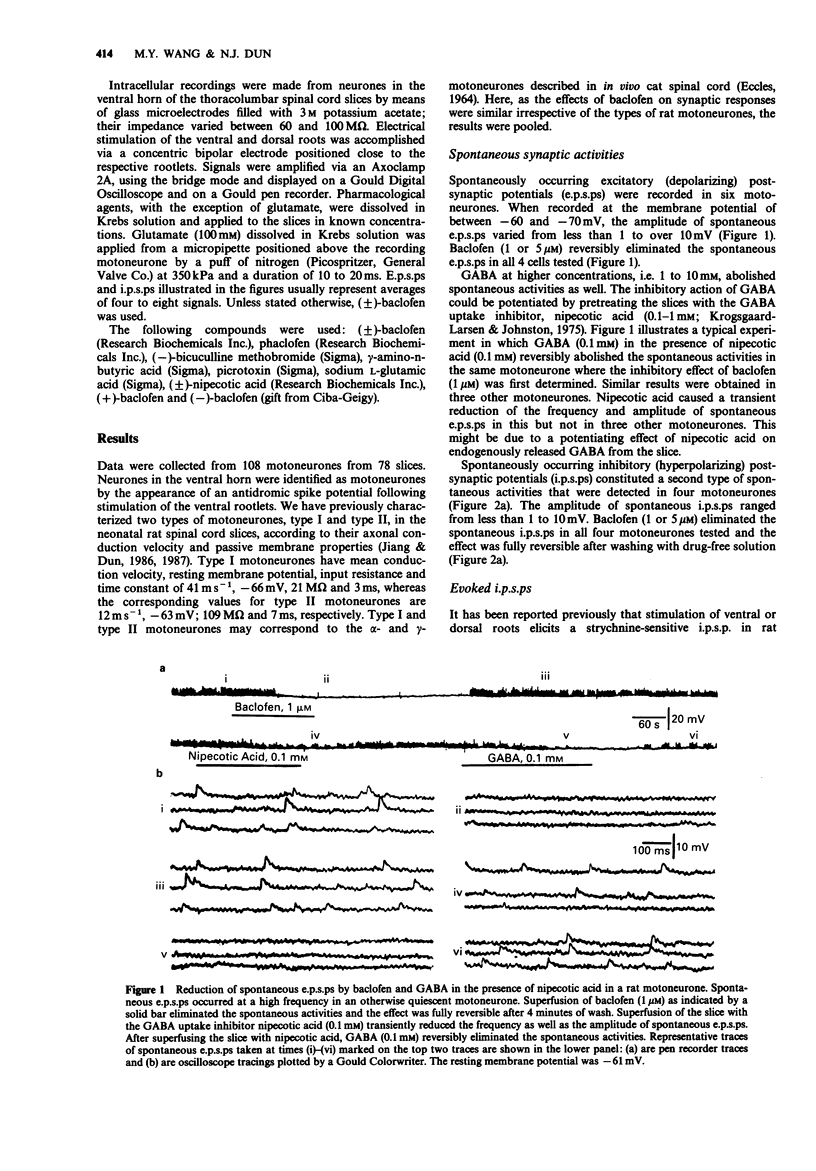
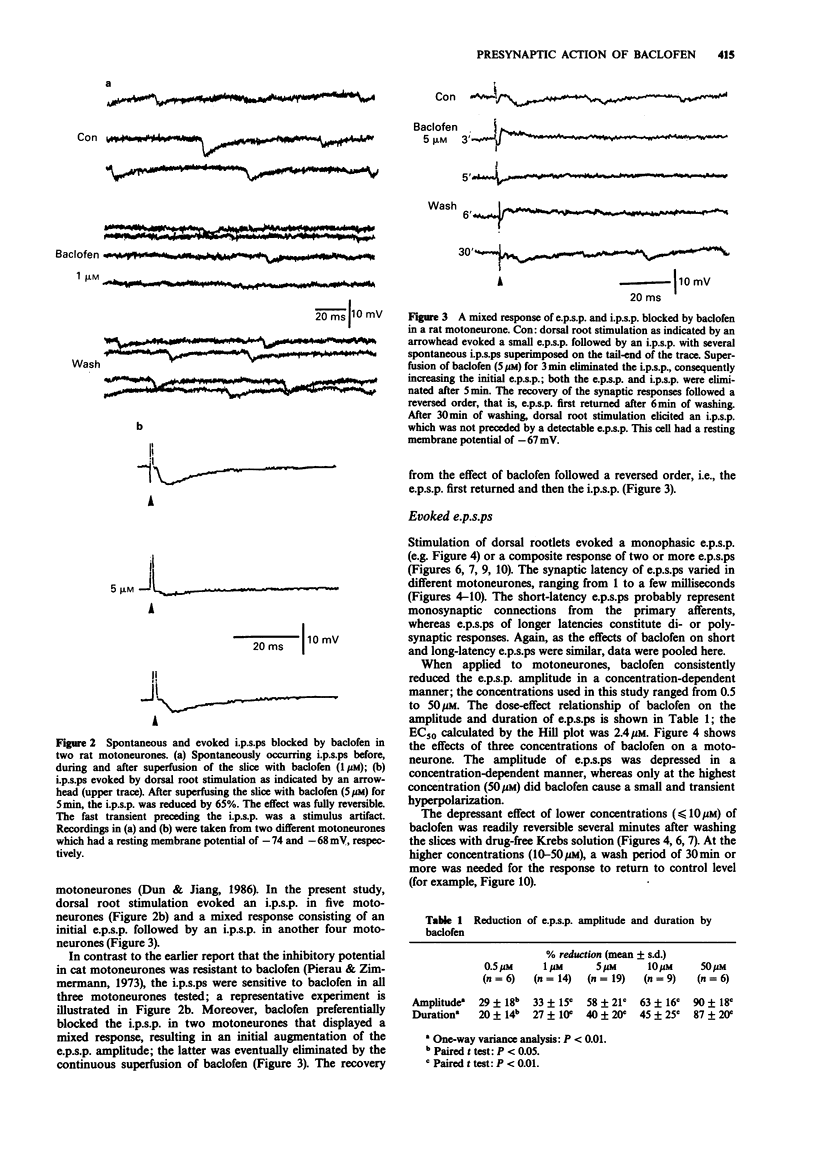
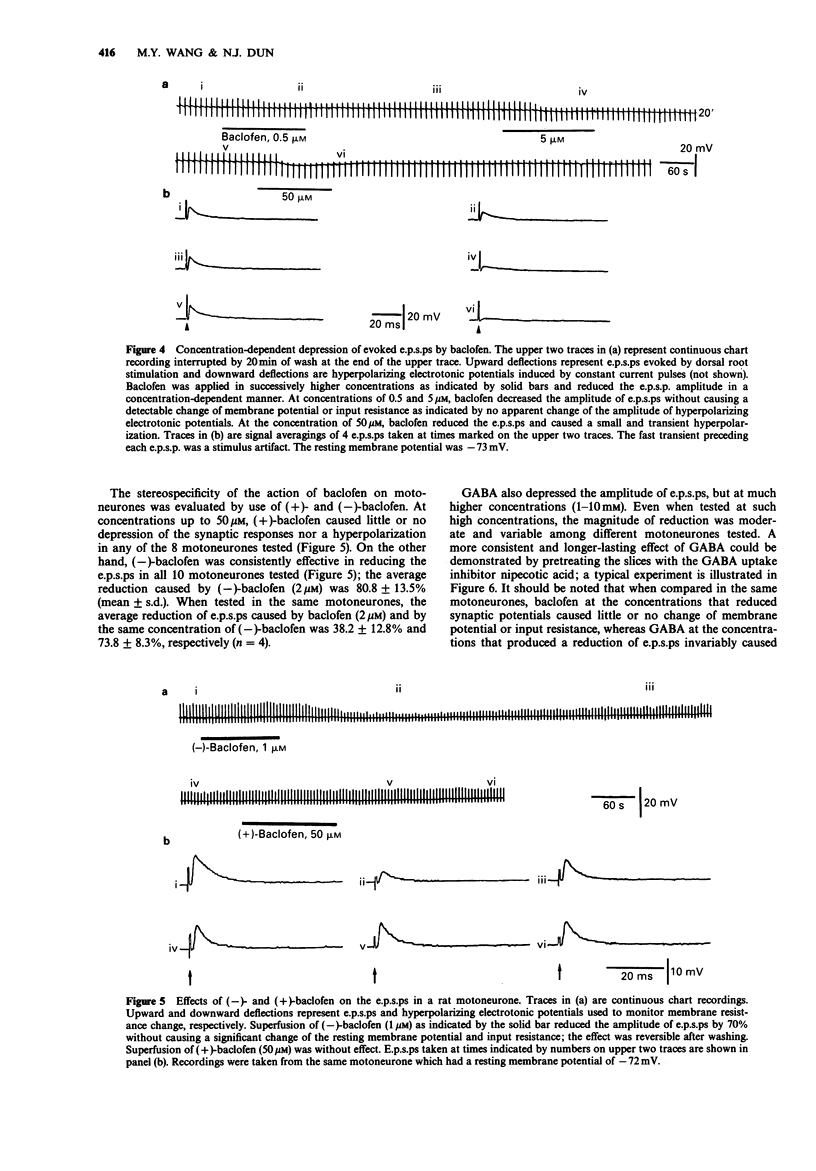
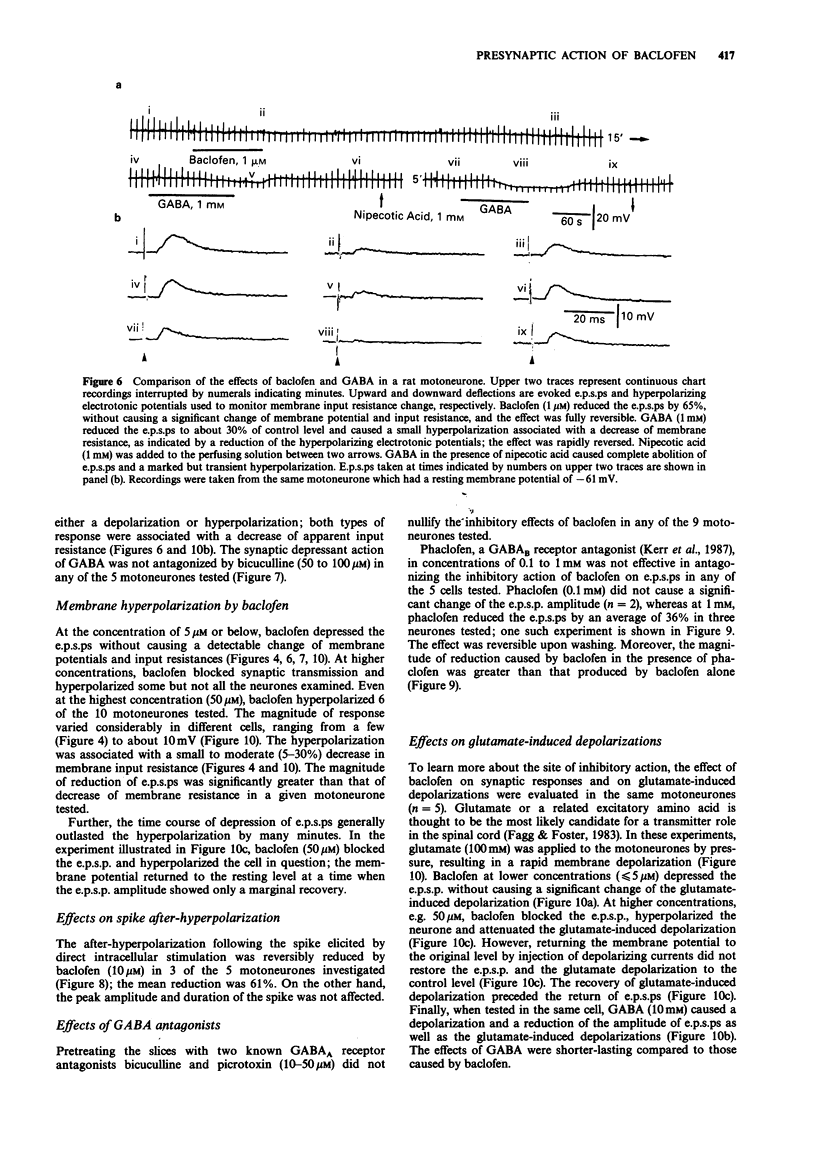
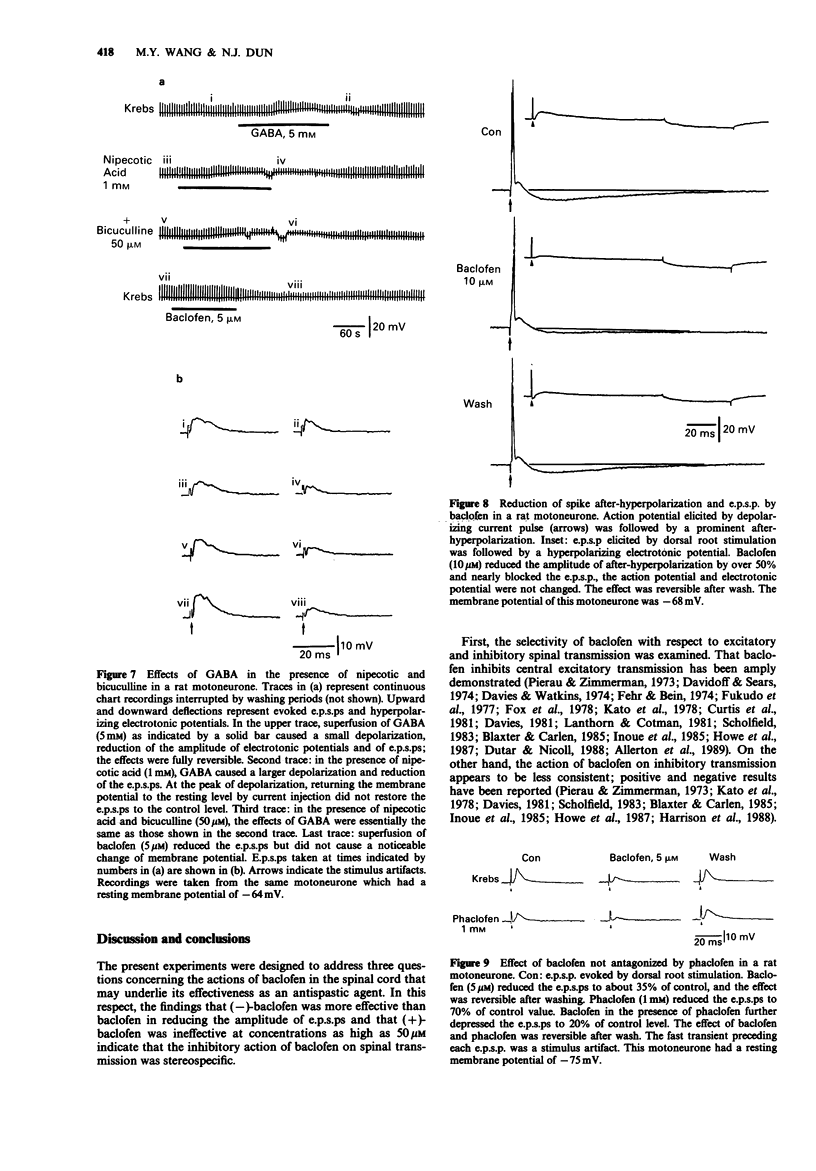
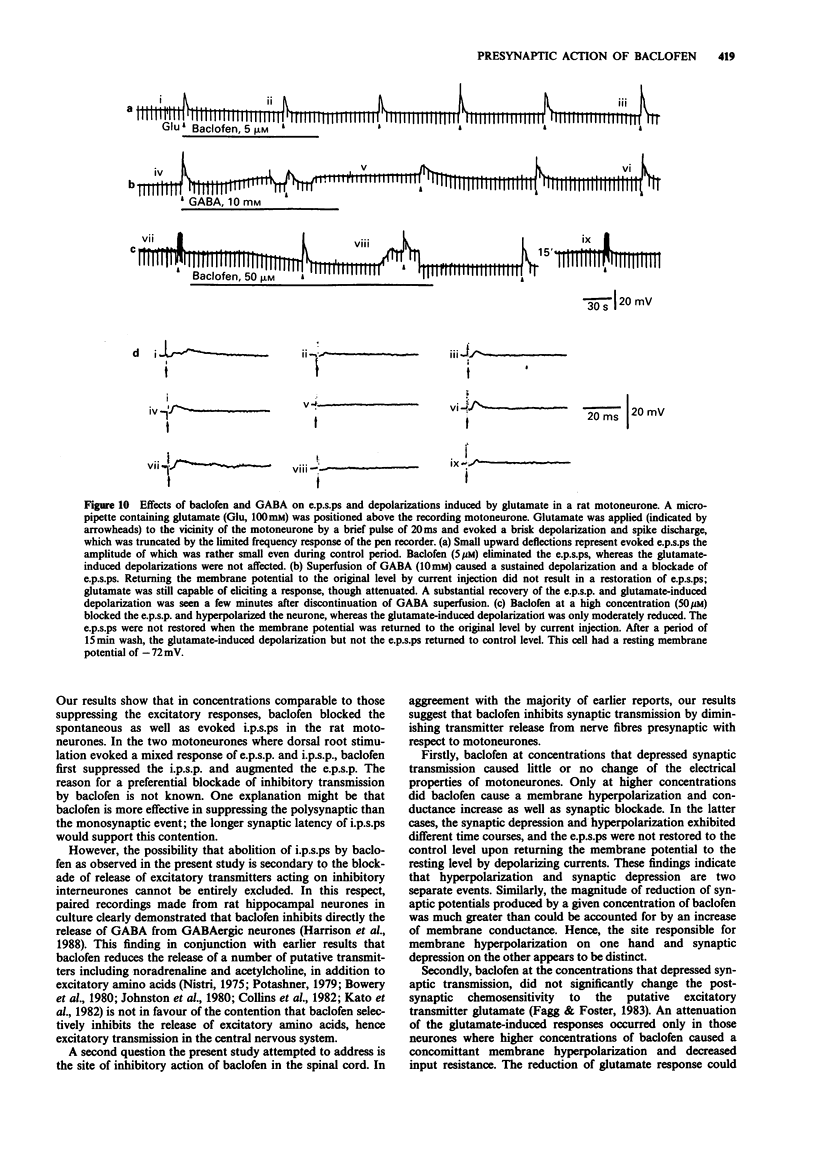
1. Intracellular recordings were made from antidromically identified motoneurones in transverse spinal cord slices from neonatal (12-16 day) rats. 2. Superfusion of (+/-)-baclofen (0.5-50 microM) reduced the excitatory postsynaptic potentials (e.p.s.ps) and inhibitory postsynaptic potentials (i.p.s.ps) evoked by dorsal root or dorsal root entry zone stimulation in a concentration-dependent manner; the calculated EC50 was 2.4 microM. Baclofen in comparable concentrations also reversibly eliminated spontaneously occurring e.p.s.ps and i.p.s.ps. 3. (-)-Baclofen was more effective as compared to baclofen in reducing the synaptic responses, whereas (+)-baclofen at concentrations as high as 50 microM was ineffective. 4. Baclofen (less than 5 microM) attenuated the synaptic responses without causing a significant change of passive membrane properties and depolarizations induced by exogenously applied glutamate. In addition to synaptic depression, baclofen (greater than 5 microM) caused a hyperpolarization associated with decreased membrane resistance in some of the motoneurones; the glutamate responses were also attenuated. 5. Baclofen reversibly depressed the spike after-hyperpolarization of the motoneurones. 6. GABA (1-10 mM) depressed synaptic transmission and depolarized or hyperpolarized motoneurones. While potentiated by the uptake inhibitor nipecotic acid, the synaptic depressant effect of GABA was not antagonized by bicuculline. 7. The synaptic depressant effect of baclofen was neither blocked by GABAA antagonists bicuculline and picrotoxin (10-50 microM) nor by the GABAB antagonist phaclofen (0.1-1 mM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allerton C. A., Boden P. R., Hill R. G. Actions of the GABAB agonist, (-)-baclofen, on neurones in deep dorsal horn of the rat spinal cord in vitro. Br J Pharmacol. 1989 Jan;96(1):29–38. doi: 10.1111/j.1476-5381.1989.tb11780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter T. J., Carlen P. L. Pre- and postsynaptic effects of baclofen in the rat hippocampal slice. Brain Res. 1985 Aug 19;341(1):195–199. doi: 10.1016/0006-8993(85)91489-1. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Anson J., Kelly E. P. Baclofen: effects on evoked field potentials and amino acid neurotransmitter release in the rat olfactory cortex slice. Brain Res. 1982 Apr 29;238(2):371–383. doi: 10.1016/0006-8993(82)90111-1. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Johnston G. A., McCulloch R. M. Central effects of beta-(para-chlorophenyl)-gamma-aminobutyric acid. Brain Res. 1974 Apr 26;70(3):493–499. doi: 10.1016/0006-8993(74)90257-1. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Bornstein J. C., Peet M. J. Selective effects of (-)-baclofen on spinal synaptic transmission in the cat. Exp Brain Res. 1981;42(2):158–170. doi: 10.1007/BF00236902. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Sears E. S. The effects of Lioresal on synaptic activity in the isolated spinal cord. Neurology. 1974 Oct;24(10):957–963. doi: 10.1212/wnl.24.10.957. [DOI] [PubMed] [Google Scholar]
- Davies J. Selective depression of synaptic excitation in cat spinal neurones by baclofen: an iontophoretic study. Br J Pharmacol. 1981 Feb;72(2):373–384. doi: 10.1111/j.1476-5381.1981.tb09137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. The action of beta-phenyl-GABA derivatives on neurones of the cat cerebral cortex. Brain Res. 1974 Apr 26;70(3):501–505. doi: 10.1016/0006-8993(74)90258-3. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Mo N. Inhibitory postsynaptic potentials in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1989 Mar;410:267–281. doi: 10.1113/jphysiol.1989.sp017532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Foster A. C. Amino acid neurotransmitters and their pathways in the mammalian central nervous system. Neuroscience. 1983 Aug;9(4):701–719. doi: 10.1016/0306-4522(83)90263-4. [DOI] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Kudo Y., Ono H. Effects of beta-(p-chlorophenyl)-GABA (baclofen) on spinal synaptic activity. Eur J Pharmacol. 1977 Jul 1;44(1):17–24. doi: 10.1016/0014-2999(77)90111-x. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lange G. D., Barker J. L. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neurosci Lett. 1988 Feb 15;85(1):105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Hasuo H., Gallagher J. P. Comparison of antagonism by phaclofen of baclofen induced hyperpolarizations and synaptically mediated late hyperpolarizing potentials recorded intracellularly from rat dorsolateral septal neurons. Neurosci Lett. 1988 Mar 21;86(1):77–81. doi: 10.1016/0304-3940(88)90186-3. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Characterization of pre- and postsynaptic actions of (-)-baclofen in the guinea-pig hippocampus in vitro. Br J Pharmacol. 1985 Apr;84(4):843–851. doi: 10.1111/j.1476-5381.1985.tb17378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. G., Dun N. J. Presynaptic suppression of excitatory postsynaptic potentials in rat ventral horn neurons by muscarinic agonists. Brain Res. 1986 Aug 27;381(1):182–186. doi: 10.1016/0006-8993(86)90710-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Hailstone M. H., Freeman C. G. Baclofen: stereoselective inhibition of excitant amino acid release. J Pharm Pharmacol. 1980 Mar;32(3):230–231. doi: 10.1111/j.2042-7158.1980.tb12902.x. [DOI] [PubMed] [Google Scholar]
- Kato K., Goto M., Fukuda H. Baclofen: inhibition of the release of L-[3H]glutamate and L-[3H]aspartate from rat whole brain synaptosomes. Gen Pharmacol. 1982;13(5):445–447. doi: 10.1016/0306-3623(82)90112-4. [DOI] [PubMed] [Google Scholar]
- Kato M., Waldmann U., Murakami S. Effects of baclofen on spinal neurones of cats. Neuropharmacology. 1978 Oct;17(10):827–833. doi: 10.1016/0028-3908(78)90071-0. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Johnston G. A. Inhibition of GABA uptake in rat brain slices by nipecotic acid, various isoxazoles and related compounds. J Neurochem. 1975 Dec;25(6):797–802. doi: 10.1111/j.1471-4159.1975.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- Nistri A. Further investigations into the effects of baclofen (Lioresal) on the isolated spinal cord. Experientia. 1975 Sep 15;31(9):1066–1068. doi: 10.1007/BF02326963. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release. Can J Physiol Pharmacol. 1978 Feb;56(1):150–154. doi: 10.1139/y78-019. [DOI] [PubMed] [Google Scholar]
- Robertson B., Taylor W. R. Effects of gamma-aminobutyric acid and (-)-baclofen on calcium and potassium currents in cat dorsal root ganglion neurones in vitro. Br J Pharmacol. 1986 Dec;89(4):661–672. doi: 10.1111/j.1476-5381.1986.tb11170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. Baclofen blocks postsynaptic inhibition but not the effect of muscimol in the olfactory cortex. Br J Pharmacol. 1983 Jan;78(1):79–84. doi: 10.1111/j.1476-5381.1983.tb09365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


