Abstract
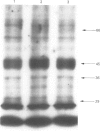
1. Prostacyclin and adenosine A2 receptors activate adenylate cyclase in the neuroblastoma hybrid cell lines NG108-15 and NCB-20. Prolonged exposure of NG108-15 cells to iloprost (a stable analogue of prostacyclin) results in a subsequent reduction in the capacity for adenylate cyclase activation by iloprost, the adenosine analogue 5'-(N-ethyl)-carboxamidoadenosine (NECA) or NaF. In contrast prolonged exposure of NCB-20 cells to iloprost results only in the loss of iloprost responsiveness. 2. Iloprost pretreatment of NG108-15 cells also magnified the morphine-dependent inhibition of iloprost-stimulated adenylate cyclase activity from 36 to 48%. This change was not due to lower iloprost stimulation following desensitization, since the % inhibition of adenylate cyclase activity by morphine in control cells was constant irrespective of enzyme activity. 3. These heterologous effects observed in NG108-15 cells following iloprost pretreatment may involve changes in the GS alpha protein, since there was a reduction of about 30% in the cholera toxin-induced [32P]-ADP-ribosylation of a 45 kDa protein from cell membranes (corresponding to the extent of loss of NECA or NaF responsiveness). A similar reduction was not observed in NCB-20 cells. 4. These results indicate that iloprost pretreatment induces different forms of desensitization in NG108-15 and NCB-20 cell lines. The heterologous desensitization in the former may, like the human platelet, involve a functional loss of GS alpha from the cell membrane. Changes in the activity of GS alpha may also account for the heterologous effects on receptors that mediate inhibition of adenylate cyclase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair I. A., Leigh P. J., MacDermot J. Desensitization of prostacyclin receptors in a neuronal hybrid cell line. Br J Pharmacol. 1982 Sep;77(1):121–127. doi: 10.1111/j.1476-5381.1982.tb09277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Butcher R. W. Desensitization of adenylate cyclase in cultured fibroblasts with prostaglandin E1 and epinephrine. J Biol Chem. 1979 Oct 10;254(19):9373–9378. [PubMed] [Google Scholar]
- Edwards R. J., MacDermot J., Wilkins A. J. Prostacyclin analogues reduce ADP-ribosylation of the alpha-subunit of the regulatory Gs-protein and diminish adenosine (A2) responsiveness of platelets. Br J Pharmacol. 1987 Mar;90(3):501–510. doi: 10.1111/j.1476-5381.1987.tb11199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity M. J., Andreasen T. J., Storm D. R., Robertson R. P. Prostaglandin E-induced heterologous desensitization of hepatic adenylate cyclase. Consequences on the guanyl nucleotide regulatory complex. J Biol Chem. 1983 Jul 25;258(14):8692–8697. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Hopkins N. K. Agonist-specific desensitization of PGI2-stimulated cyclic AMP accumulation by PGE1 in human foreskin fibroblasts. Prostaglandins. 1980 Jan;19(1):2–16. doi: 10.1016/0090-6980(80)90148-3. [DOI] [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Haslam R. J., Rosson G. M. Effects of adenosine on levels of adenosine cyclic 3',5'-monophosphate in human blood platelets in relation to adenosine incorporation and platelet aggregation. Mol Pharmacol. 1975 Sep;11(5):528–544. [PubMed] [Google Scholar]
- Hsia J. A., Hewlett E. L., Moss J. Heterologous desensitization of adenylate cyclase with prostaglandin E1 alters sensitivity to inhibitory as well as stimulatory agonists. J Biol Chem. 1985 Apr 25;260(8):4922–4926. [PubMed] [Google Scholar]
- Hsia J. A., Moss J., Hewlett E. L., Vaughan M. Requirement for both choleragen and pertussis toxin to obtain maximal activation of adenylate cyclase in cultured cells. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1068–1074. doi: 10.1016/0006-291x(84)90883-0. [DOI] [PubMed] [Google Scholar]
- Hüttemann E., Ukena D., Lenschow V., Schwabe U. Ra adenosine receptors in human platelets. Characterization by 5'-N-ethylcarboxamido[3H]adenosine binding in relation to adenylate cyclase activity. Naunyn Schmiedebergs Arch Pharmacol. 1984 Mar;325(3):226–233. doi: 10.1007/BF00495948. [DOI] [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin C., Thibout H., Lambert B., Correze C. Endogenous ADP-ribosylation of Gs subunit and autonomous regulation of adenylate cyclase. Nature. 1986 Sep 11;323(6084):182–184. doi: 10.1038/323182a0. [DOI] [PubMed] [Google Scholar]
- Jaschonek K., Faul C., Schmidt H., Renn W. Desensitization of platelets to iloprost. Loss of specific binding sites and heterologous desensitization of adenylate cyclase. Eur J Pharmacol. 1988 Mar 1;147(2):187–196. doi: 10.1016/0014-2999(88)90777-7. [DOI] [PubMed] [Google Scholar]
- Kassis S., Fishman P. H. Different mechanisms of desensitization of adenylate cyclase by isoproterenol and prostaglandin E1 in human fibroblasts. Role of regulatory components in desensitization. J Biol Chem. 1982 May 10;257(9):5312–5318. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Kenimer J. G., Nirenberg M. Desensitization of adenylate cyclase to prostaglandin E1 or 2-chloroadenosine. Mol Pharmacol. 1981 Nov;20(3):585–591. [PubMed] [Google Scholar]
- Leigh P. J., MacDermot J. Desensitization of prostacyclin responsiveness in a neuronal hybrid cell line: selective loss of high affinity receptors. Br J Pharmacol. 1985 May;85(1):237–247. doi: 10.1111/j.1476-5381.1985.tb08852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermot J. Desensitization of prostacyclin responsiveness in platelets. Apparent differences in the mechanism in vitro or in vivo. Biochem Pharmacol. 1986 Aug 15;35(16):2645–2649. doi: 10.1016/0006-2952(86)90169-3. [DOI] [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. V., Gorman R. R. Evidence for distinct prostaglandin I2 and D2 receptors in human platelets. J Pharmacol Exp Ther. 1979 Jul;210(1):134–140. [PubMed] [Google Scholar]
- Milligan G., McKenzie F. R. Opioid peptides promote cholera-toxin-catalysed ADP-ribosylation of the inhibitory guanine-nucleotide-binding protein (Gi) in membranes of neuroblastoma x glioma hybrid cells. Biochem J. 1988 Jun 1;252(2):369–373. doi: 10.1042/bj2520369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Rich K. A., Codina J., Floyd G., Sekura R., Hildebrandt J. D., Iyengar R. Glucagon-induced heterologous desensitization of the MDCK cell adenylyl cyclase. Increases in the apparent levels of the inhibitory regulator (Ni). J Biol Chem. 1984 Jun 25;259(12):7893–7901. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Sinzinger H., Silberbauer K., Horsch A. K., Gall A. Decreased sensitivity of human platelets to PGI2 during long-term intraarterial prostacyclin infusion in patients with peripheral vascular disease--a rebound phenomenon? Prostaglandins. 1981 Jan;21(1):49–51. doi: 10.1016/0090-6980(81)90195-7. [DOI] [PubMed] [Google Scholar]
- Skuballa W., Vorbrüggen H. Synthesis of ciloprost (ZK 36 374): a chemically stable and biologically potent prostacyclin analog. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:299–305. [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Liao Y. C., Alborzi A., Beiderman B., Chang F. H., Masters S. B., Levinson A. D., Bourne H. R. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]