Abstract
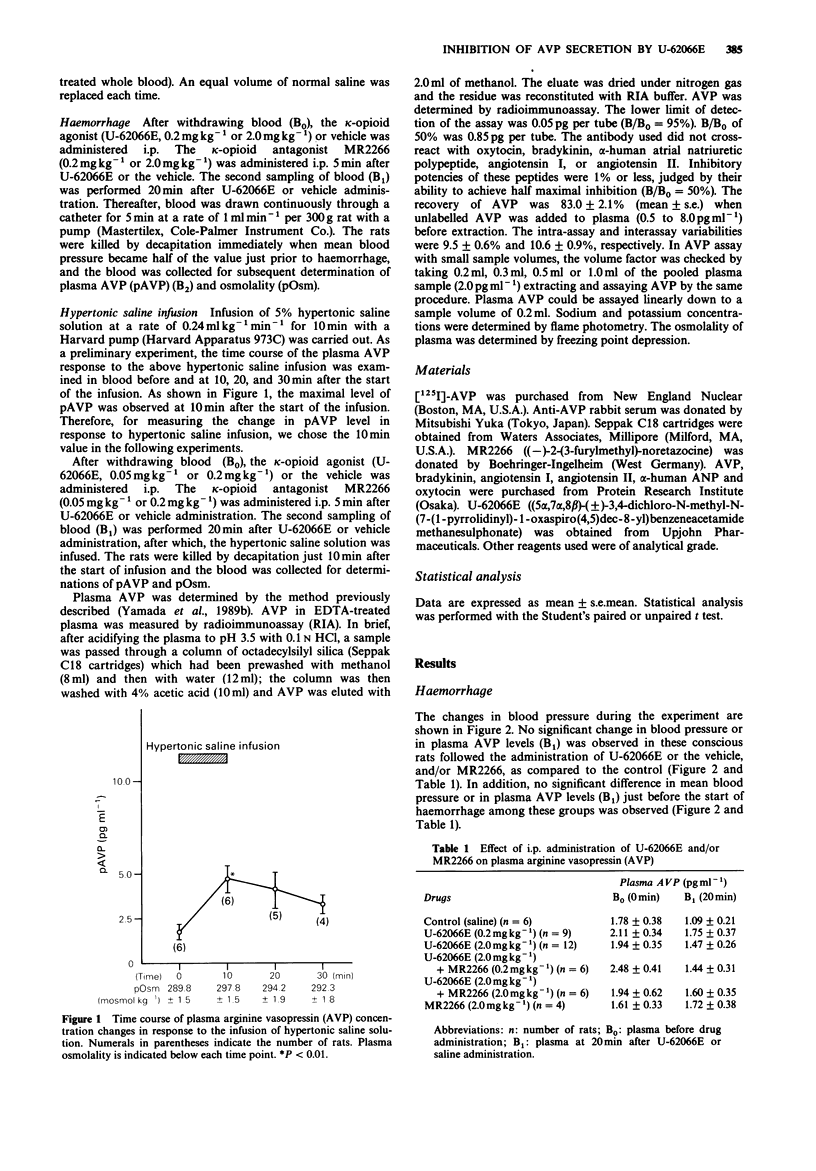
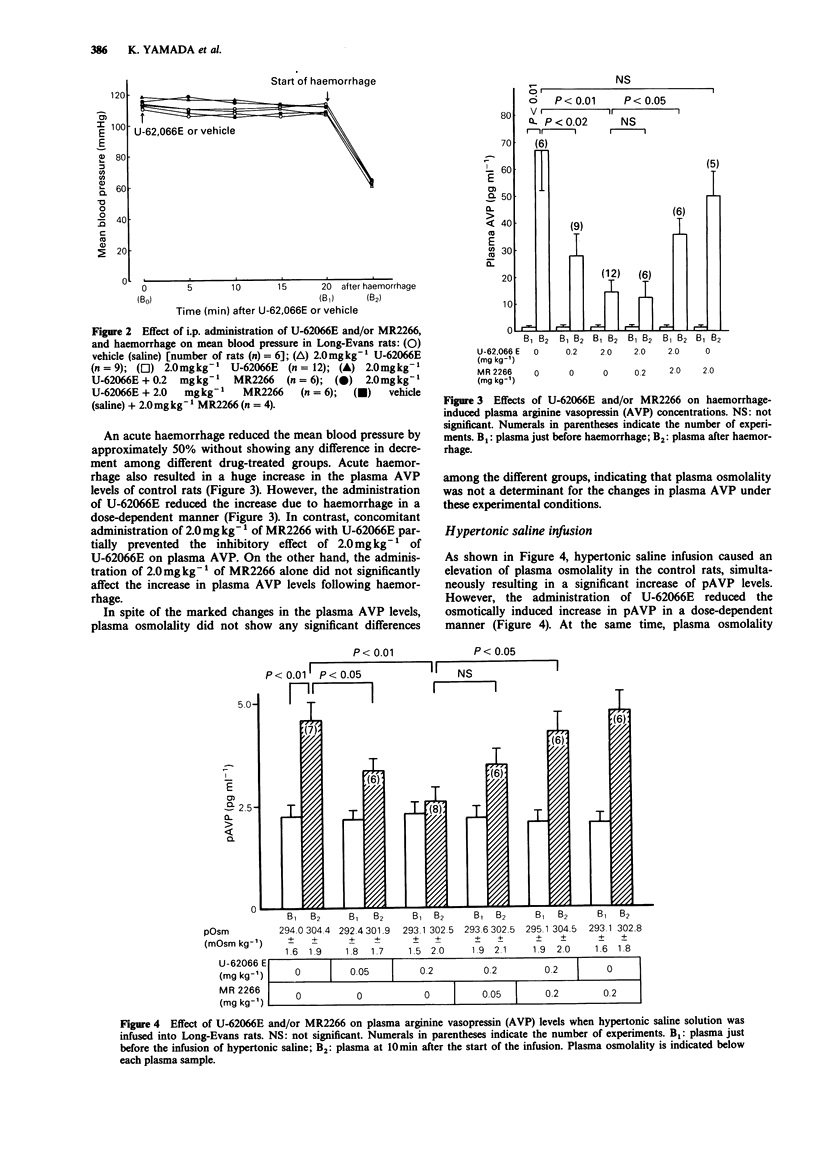
1. The effect of kappa (kappa) opioid receptor activation on the increase in arginine vasopressin (AVP) secretion evoked by two acute and quite different stimuli (i.e., haemorrhage and osmotic stimulus due to hypertonic saline infusion) were evaluated in conscious Long-Evans rats, by use of U-62066E, a highly selective kappa-opioid receptor agonist, and MR2266, an opioid receptor antagonist with some selectivity for kappa-receptors. 2. An acute haemorrhage, which reduced the mean blood pressure by approximately 50%, resulted in a large increase in the plasma AVP (pAVP) levels of control rats. However, the administration of U-62066E (0.2 mg kg-1 or 2.0 mg kg-1) reduced the increase due to haemorrhage in a dose-dependent manner. In contrast, concomitant administration of 2.0 mg kg-1 of MR2266 with U-62066E significantly attenuated the inhibition of pAVP levels produced by U-62066E 2.0 mg kg-1. 3. Hypertonic saline infusion (5% hypertonic saline solution at a rate of 0.24 ml kg-1 min-1 for 10 min) caused the elevation of plasma osmolality (pOsm) from 294.0 +/- 1.6 mosmol kg-1 to 304.4 +/- 1.9 mosmol kg-1, simultaneously resulting in a significant increase in pAVP levels from 2.34 +/- 0.28 pg ml-1 to 4.54 +/- 0.51 pg ml-1. However, the administration of U-62066E (0.05 mg kg-1 or 0.2 mg kg-1) reduced the osmotically induced increase in pAVP in a dose-dependent manner although pOsm showed the same degree of increase as in controls.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenbrey G. A., Handelman W. A., Arnold P., Manning M., Schrier R. W. Vascular effects of arginine vasopressin during fluid deprivation in the rat. J Clin Invest. 1981 Apr;67(4):961–968. doi: 10.1172/JCI110146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. A., Lightman S. L. Selective cardiovascular and neuroendocrine effects of a kappa-opioid agonist in the nucleus tractus solitarii of rats. J Physiol. 1985 Oct;367:363–375. doi: 10.1113/jphysiol.1985.sp015829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C., Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Wood P., Merrick L., Lincoln D. W. Opiate inhibition of peptide release from the neurohumoral terminals of hypothalamic neurones. Nature. 1979 Dec 13;282(5740):746–748. doi: 10.1038/282746a0. [DOI] [PubMed] [Google Scholar]
- Faden A. I., Feuerstein G. Hypothalamic regulation of the cardiovascular and respiratory systems: role of specific opiate receptors. Br J Pharmacol. 1983 Aug;79(4):997–1002. doi: 10.1111/j.1476-5381.1983.tb10547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke N., Martin R. Opioid binding in a rat neurohypophysial fraction enriched in oxytocin and vasopressin nerve endings. Neurosci Lett. 1985 Oct 24;61(1-2):37–41. doi: 10.1016/0304-3940(85)90397-0. [DOI] [PubMed] [Google Scholar]
- GINSBURG M., HELLER H. Antidiuretic activity in blood obtained from various parts of the cardiovascular system. J Endocrinol. 1953 Jul;9(3):274–282. doi: 10.1677/joe.0.0090274. [DOI] [PubMed] [Google Scholar]
- Gordon F. J. Central opioid receptors and baroreflex control of sympathetic and cardiovascular function. J Pharmacol Exp Ther. 1986 May;237(2):428–436. [PubMed] [Google Scholar]
- Herkenham M., Rice K. C., Jacobson A. E., Rothman R. B. Opiate receptors in rat pituitary are confined to the neural lobe and are exclusively kappa. Brain Res. 1986 Sep 24;382(2):365–371. doi: 10.1016/0006-8993(86)91346-6. [DOI] [PubMed] [Google Scholar]
- Jacob J. J., Ramabadran K. Opioid antagonists, endogenous ligands and nociception. Eur J Pharmacol. 1977 Dec 15;46(4):393–394. doi: 10.1016/0014-2999(77)90235-7. [DOI] [PubMed] [Google Scholar]
- Lahti R. A., Mickelson M. M., McCall J. M., Von Voigtlander P. F. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985 Feb 26;109(2):281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- Laycock J. F., Penn W., Shirley D. G., Walter S. J. The role of vasopressin in blood pressure regulation immediately following acute haemorrhage in the rat. J Physiol. 1979 Nov;296:267–275. doi: 10.1113/jphysiol.1979.sp013004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander J. D. Further study of kappa opioids on increased urination. J Pharmacol Exp Ther. 1983 Oct;227(1):35–41. [PubMed] [Google Scholar]
- Leander J. D., Hart J. C., Zerbe R. L. Kappa agonist-induced diuresis: evidence for stereoselectivity, strain differences, independence of hydration variables and a result of decreased plasma vasopressin levels. J Pharmacol Exp Ther. 1987 Jul;242(1):33–39. [PubMed] [Google Scholar]
- Mickelson M. M., Lahti R. A. 3H-SKF10047 receptor binding studies. Attempts to define the opioid sigma receptor. Neuropeptides. 1984 Dec;5(1-3):149–152. doi: 10.1016/0143-4179(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Linas S. L. Role of angiotensin II, alpha-adrenergic system, and arginine vasopressin on arterial pressure in rat. Am J Physiol. 1984 Jan;246(1 Pt 2):H25–H30. doi: 10.1152/ajpheart.1984.246.1.H25. [DOI] [PubMed] [Google Scholar]
- Pesce G., Lang M. A., Russell J. T., Rodbard D., Gainer H. Characterization of kappa opioid receptors in neurosecretosomes from bovine posterior pituitary. J Neurochem. 1987 Aug;49(2):421–427. doi: 10.1111/j.1471-4159.1987.tb02882.x. [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Berl T., Anderson R. J. Osmotic and nonosmotic control of vasopressin release. Am J Physiol. 1979 Apr;236(4):F321–F332. doi: 10.1152/ajprenal.1979.236.4.F321. [DOI] [PubMed] [Google Scholar]
- Share L. Role of cardiovascular receptors in the control of ADH release. Cardiology. 1976;61 Suppl 1:51–64. doi: 10.1159/000169792. [DOI] [PubMed] [Google Scholar]
- Slizgi G. R., Ludens J. H. Studies on the nature and mechanism of the diuretic activity of the opioid analgesic ethylketocyclazocine. J Pharmacol Exp Ther. 1982 Mar;220(3):585–591. [PubMed] [Google Scholar]
- TORVIK A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol. 1956 Nov;106(1):51–141. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Akil H., Fischli W., Goldstein A., Zimmerman E., Nilaver G., van wimersma Griedanus T. B. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982 Apr 2;216(4541):85–87. doi: 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]
- Whitnall M. H., Gainer H., Cox B. M., Molineaux C. J. Dynorphin-A-(1-8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983 Dec 9;222(4628):1137–1139. doi: 10.1126/science.6648526. [DOI] [PubMed] [Google Scholar]
- Yamada K., Hasunuma K., Shiina T., Ito K., Tamura Y., Yoshida S. Inter-relationship between urinary kallikrein-kinins and arginine vasopressin in man. Clin Sci (Lond) 1989 Jan;76(1):13–18. doi: 10.1042/cs0760013. [DOI] [PubMed] [Google Scholar]
- Yamada K., Imai M., Yoshida S. Mechanism of diuretic action of U-62,066E, a kappa opioid receptor agonist. Eur J Pharmacol. 1989 Jan 31;160(2):229–237. doi: 10.1016/0014-2999(89)90495-0. [DOI] [PubMed] [Google Scholar]
- Zamir N., Zamir D., Eiden L. E., Palkovits M., Brownstein M. J., Eskay R. L., Weber E., Faden A. I., Feuerstein G. Methionine and leucine enkephalin in rat neurohypophysis: different responses to osmotic stimuli and T2 toxin. Science. 1985 May 3;228(4699):606–608. doi: 10.1126/science.2858918. [DOI] [PubMed] [Google Scholar]
- Zerbe R. L., Henry D. P., Robertson G. L. Vasopressin response to orthostatic hypotension. Etiologic and clinical implications. Am J Med. 1983 Feb;74(2):265–271. doi: 10.1016/0002-9343(83)90625-3. [DOI] [PubMed] [Google Scholar]



