Abstract
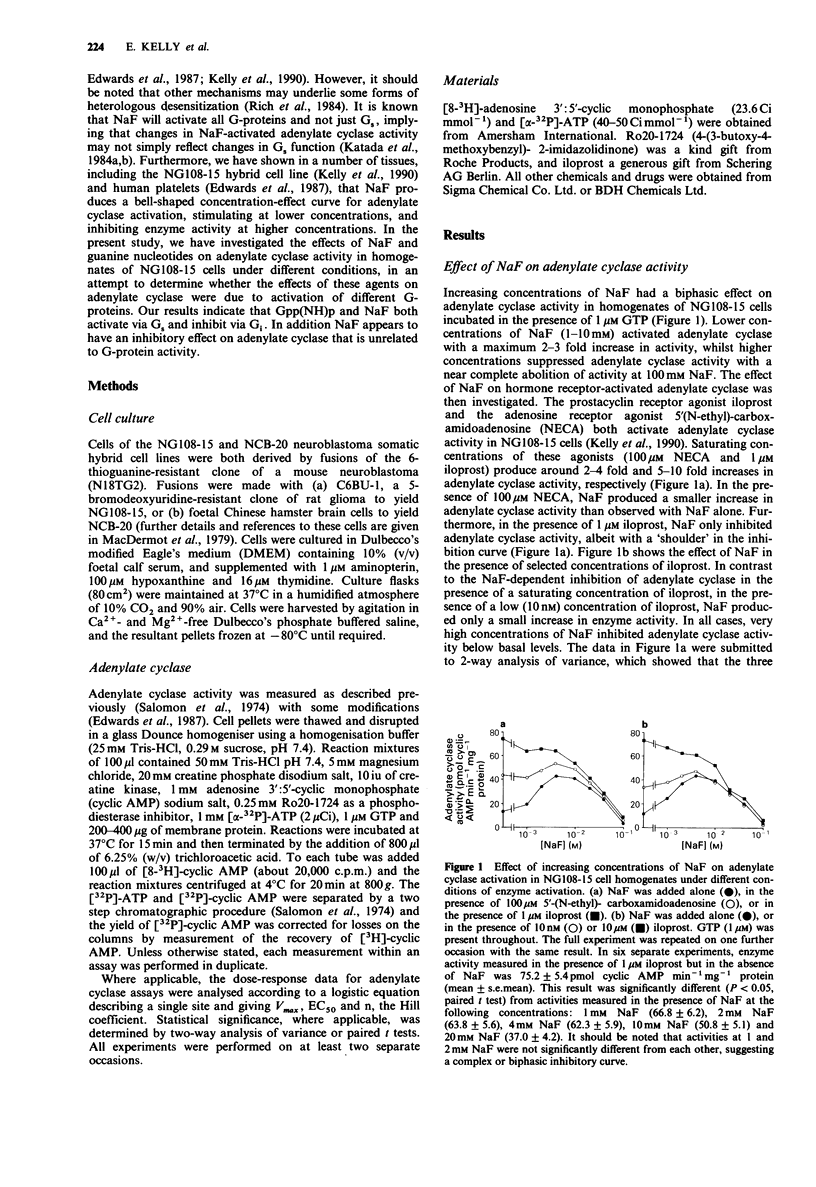
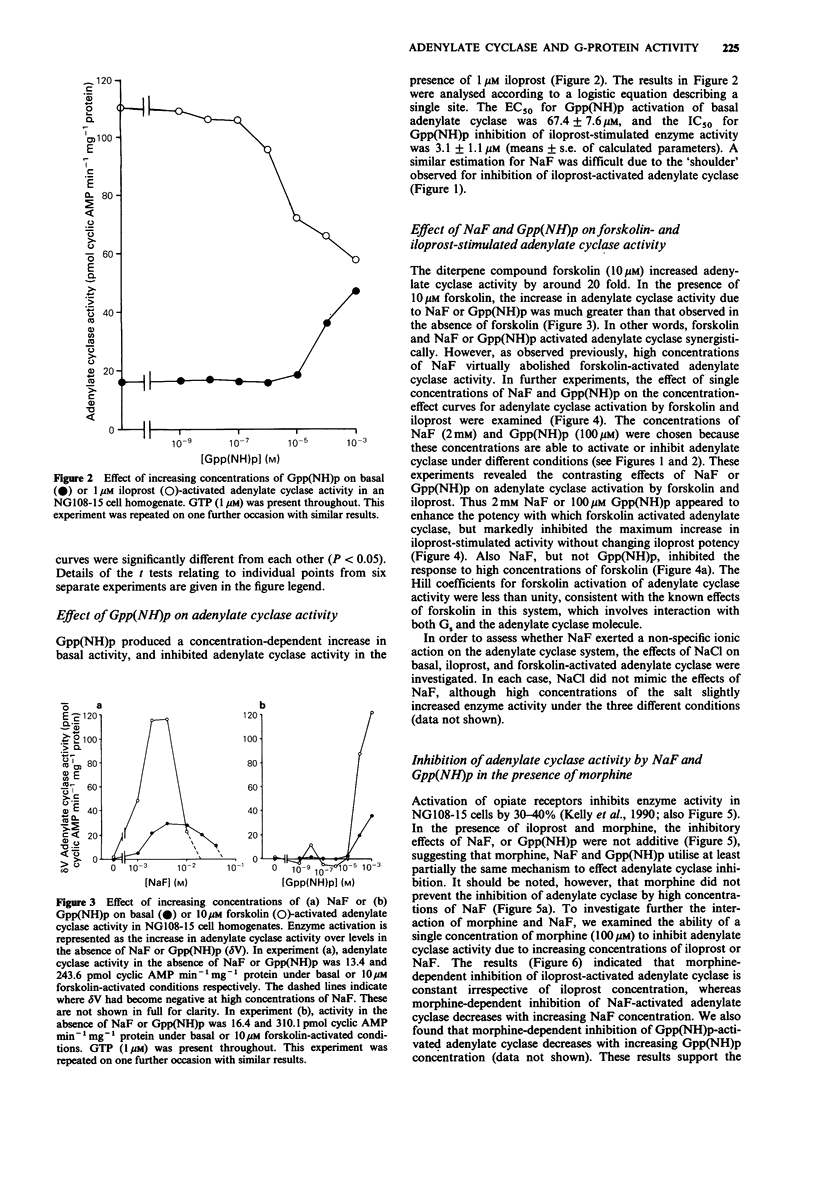
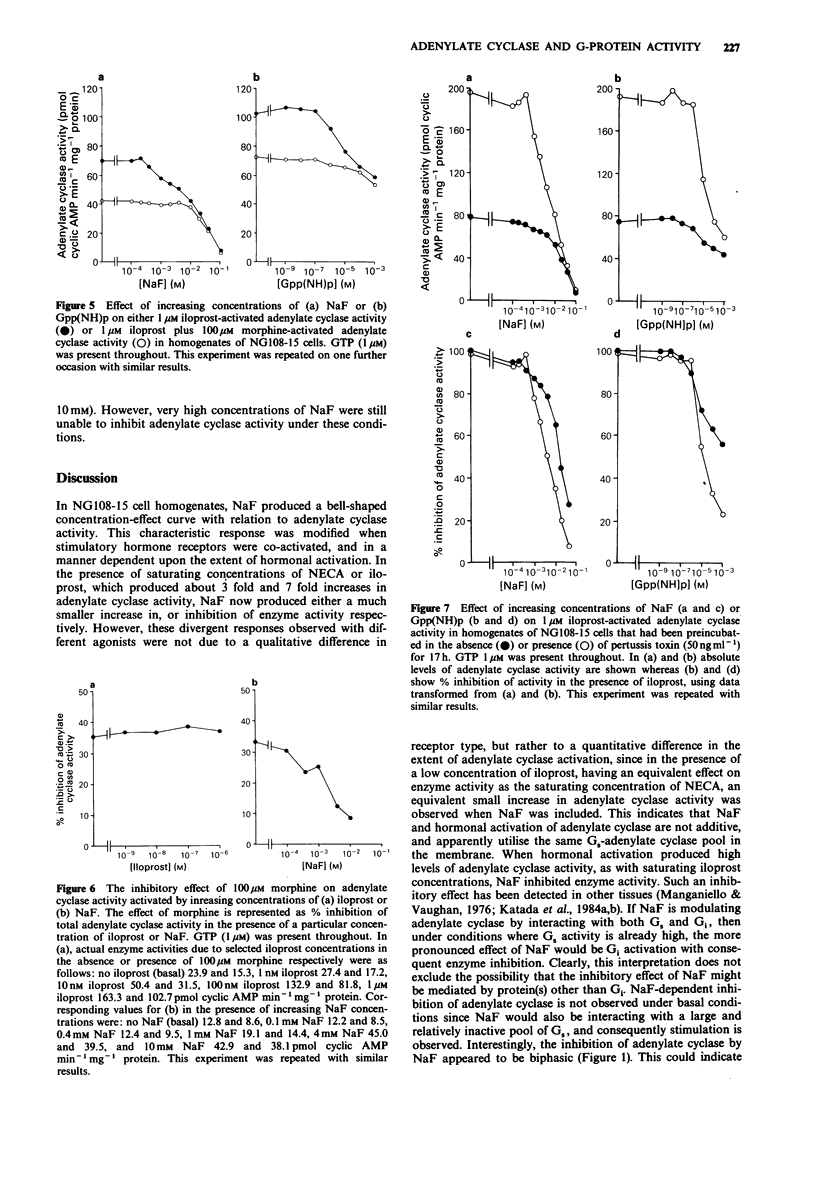
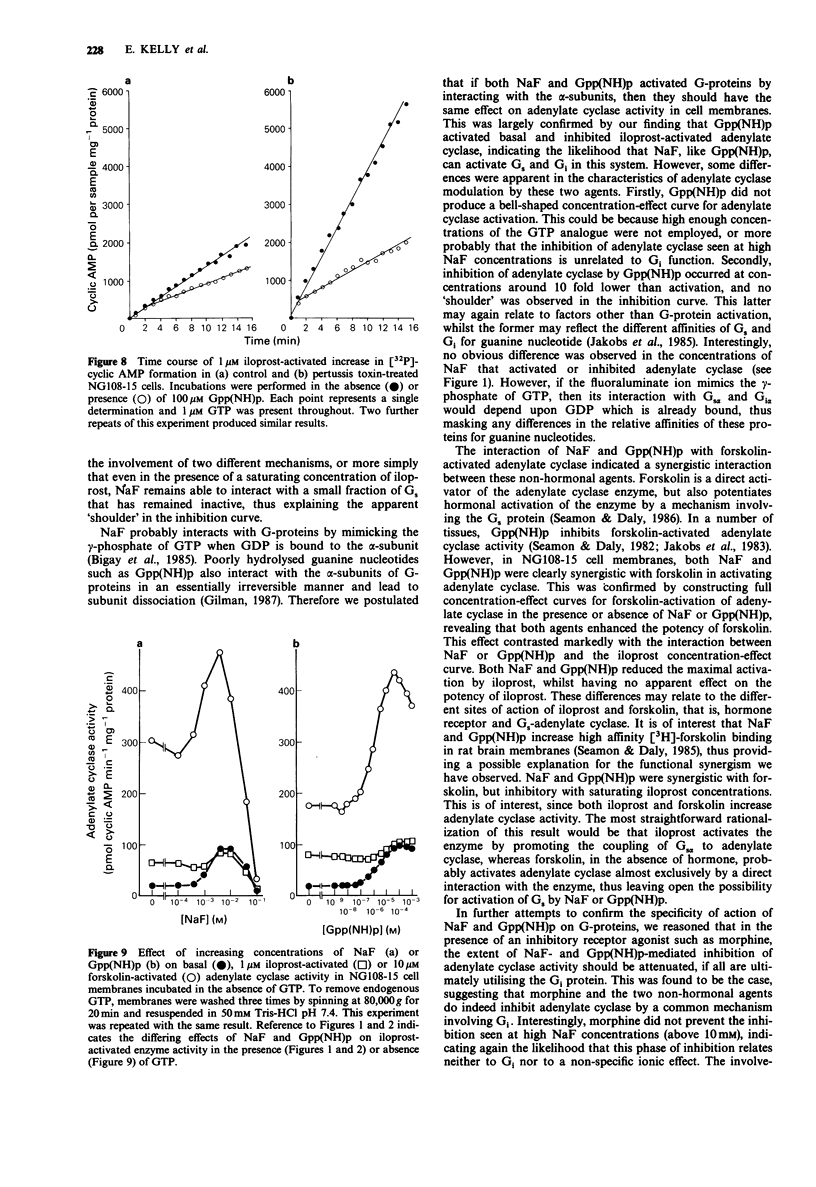
1. NaF (10 mM) produced a 2-3 fold increase in adenylate cyclase activity in homogenates of NG108-15 cells incubated in the presence of 1 microM GTP. Higher concentrations of NaF suppressed adenylate cyclase activity. 2. In the presence of the adenosine receptor agonist 5'-(N-ethyl)-carboxamidoadenosine (NECA; 100 microM) or the prostacyclin receptor agonist iloprost (10 nM), NaF produced a much smaller increase in adenylate cyclase activity, whereas in the presence of a saturating concentration of iloprost (1 microM), NaF only inhibited adenylate cyclase activity. 3. Similarly, Gpp(NH)p activated basal adenylate cyclase activity, and inhibited 1 microM iloprost-activated enzyme activity. In the presence of 10 microM forskolin, NaF or Gpp(NH)p increased adenylate cyclase activity synergistically. Analysis of concentration-effect curves indicated that NaF (2 mM) or Gpp(NH)p (100 microM) increased the potency with which forskolin activated adenylate cyclase, whilst reducing the maximum activation of adenylate cyclase by iloprost. 4. Opiate receptors mediate inhibition of adenylate cyclase, and the opiate agonist morphine (100 microM) reduced the capacity of NaF or Gpp(NH)p to inhibit iloprost-activated adenylate cyclase. Unexpectedly, pertussis toxin treatment enhanced the ability of NaF or Gpp(NH)p to inhibit iloprost-activated adenylate cyclase. 5. In the absence of GTP, NaF and Gpp(NH)p remained able both to activate basal adenylate cyclase and to be synergistic with forskolin in activating the enzyme. In contrast the ability of NaF and Gpp(NH)p to inhibit iloprost-activated adenylate cyclase was substantially lost in the absence of added GTP.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Chabre M. Aluminofluoride action on G-proteins of the adenylate cyclase system is not different from that on transducin. Biochem J. 1989 Mar 15;258(3):931–932. doi: 10.1042/bj2580931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B. Desensitization of hormonal stimuli coupled to regulation of cyclic AMP levels. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:151–209. [PubMed] [Google Scholar]
- Edwards R. J., MacDermot J., Wilkins A. J. Prostacyclin analogues reduce ADP-ribosylation of the alpha-subunit of the regulatory Gs-protein and diminish adenosine (A2) responsiveness of platelets. Br J Pharmacol. 1987 Mar;90(3):501–510. doi: 10.1111/j.1476-5381.1987.tb11199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity M. J., Andreasen T. J., Storm D. R., Robertson R. P. Prostaglandin E-induced heterologous desensitization of hepatic adenylate cyclase. Consequences on the guanyl nucleotide regulatory complex. J Biol Chem. 1983 Jul 25;258(14):8692–8697. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Godfrey P. P., Watson S. P. Fluoride inhibits agonist-induced formation of inositol phosphates in rat cortex. Biochem Biophys Res Commun. 1988 Sep 15;155(2):664–669. doi: 10.1016/s0006-291x(88)80546-1. [DOI] [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Howlett A. C., Sternweis P. C., Macik B. A., Van Arsdale P. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Association of a regulatory component of the enzyme with membranes containing the catalytic protein and beta-adrenergic receptors. J Biol Chem. 1979 Apr 10;254(7):2287–2295. [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Minuth M., Schultz G. Inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:137–150. [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Mechanism of pertussis toxin action on the adenylate cyclase system. Inhibition of the turn-on reaction of the inhibitory regulatory site. Eur J Biochem. 1984 Apr 2;140(1):177–181. doi: 10.1111/j.1432-1033.1984.tb08083.x. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Mechanisms and components involved in adenylate cyclase inhibition by hormones. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:135–143. [PubMed] [Google Scholar]
- Katada T., Amano T., Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982 Apr 10;257(7):3739–3746. [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984 Mar 25;259(6):3578–3585. [PubMed] [Google Scholar]
- Kelly E., Keen M., Nobbs P., MacDermot J. Segregation of discrete GS alpha-mediated responses that accompany homologous or heterologous desensitization in two related somatic hybrids. Br J Pharmacol. 1990 Feb;99(2):309–316. doi: 10.1111/j.1476-5381.1990.tb14700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganiello V. C., Vaughan M. Activation and inhibition of fat cell adenylate cyclase by fluoride. J Biol Chem. 1976 Oct 25;251(20):6205–6209. [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Rich K. A., Codina J., Floyd G., Sekura R., Hildebrandt J. D., Iyengar R. Glucagon-induced heterologous desensitization of the MDCK cell adenylyl cyclase. Increases in the apparent levels of the inhibitory regulator (Ni). J Biol Chem. 1984 Jun 25;259(12):7893–7901. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Guanosine 5'-(beta, gamma-imido)triphosphate inhibition of forskolin-activated adenylate cyclase is mediated by the putative inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1982 Oct 10;257(19):11591–11596. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. High-affinity binding of forskolin to rat brain membranes. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:125–135. [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Stadel J. M., Crooke S. T. Fluoride interaction with G-proteins. Biochem J. 1989 Mar 15;258(3):932–933. doi: 10.1042/bj2580932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]


