Abstract
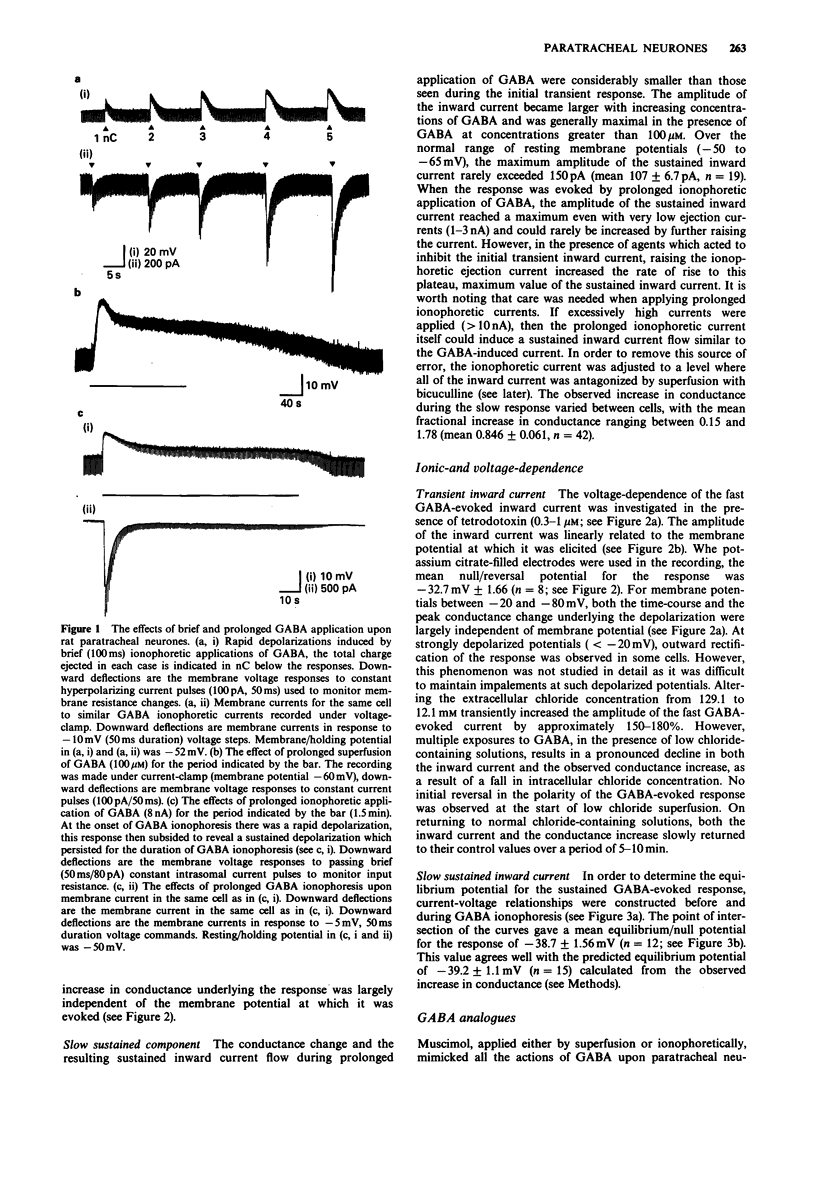
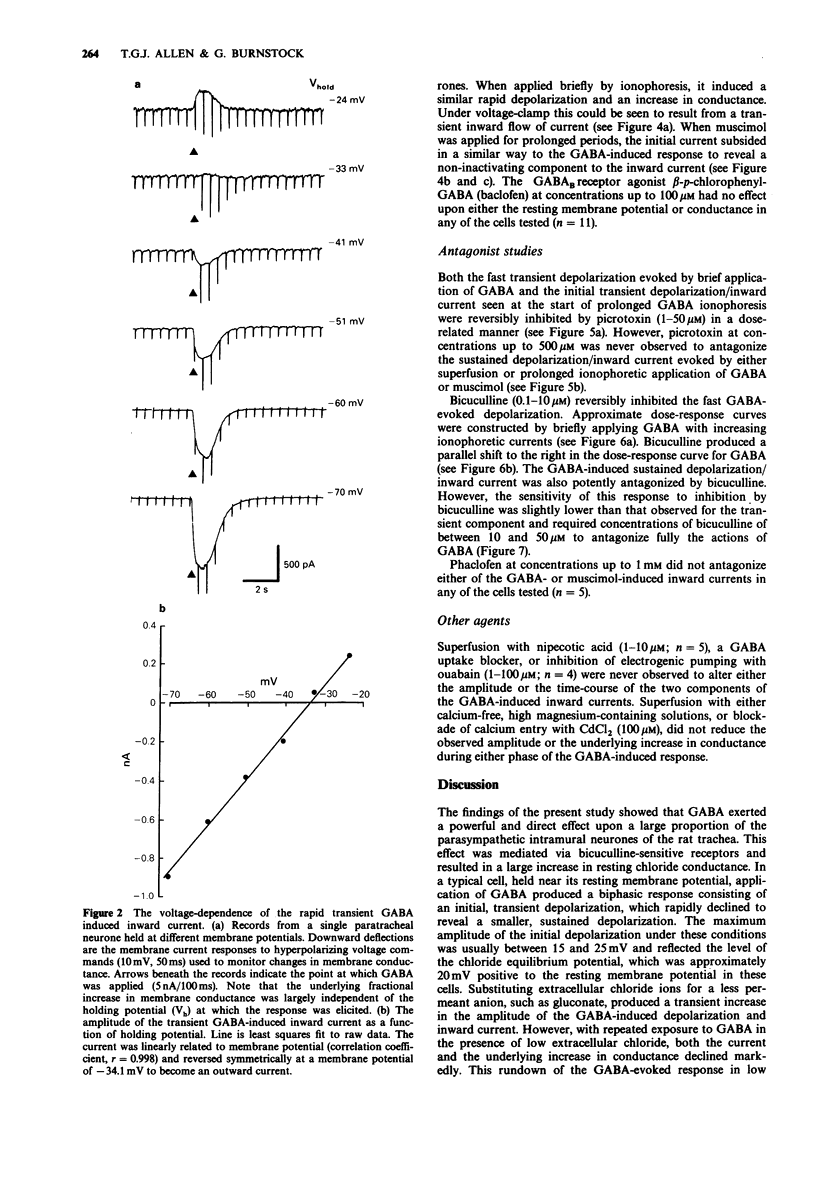
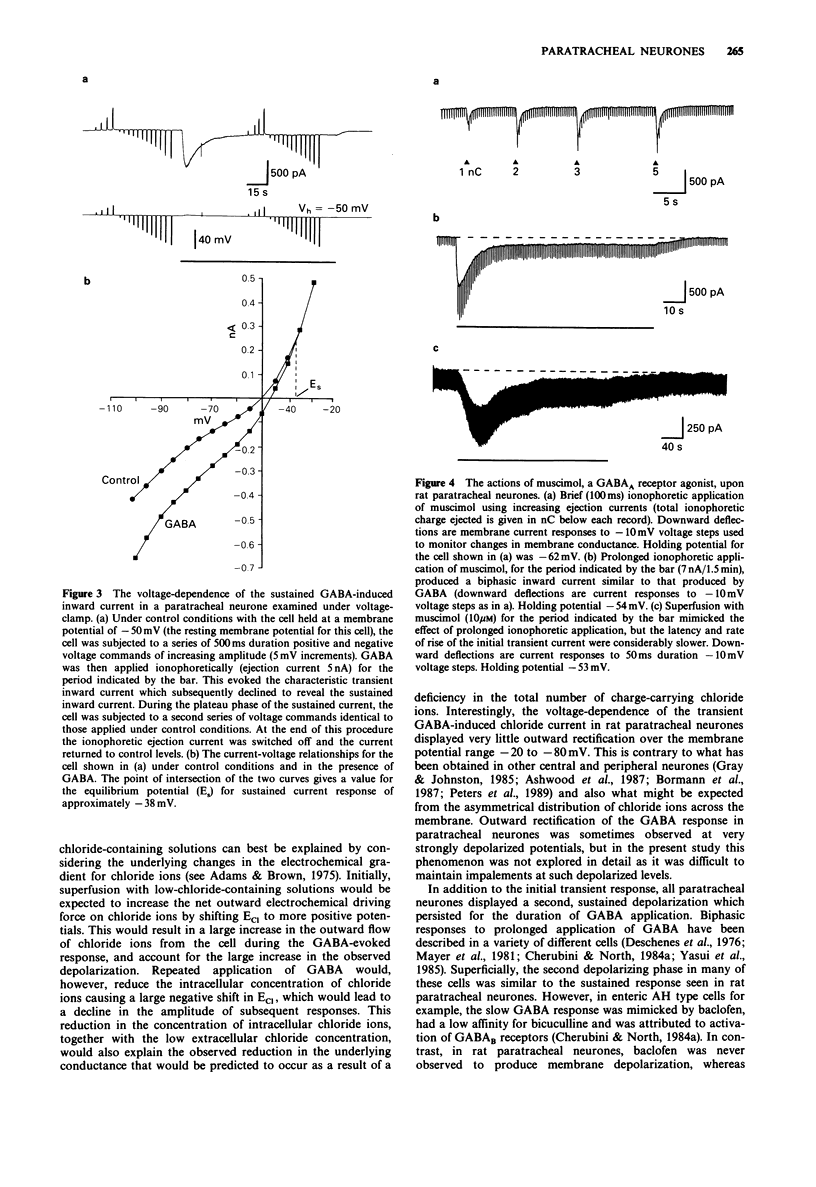
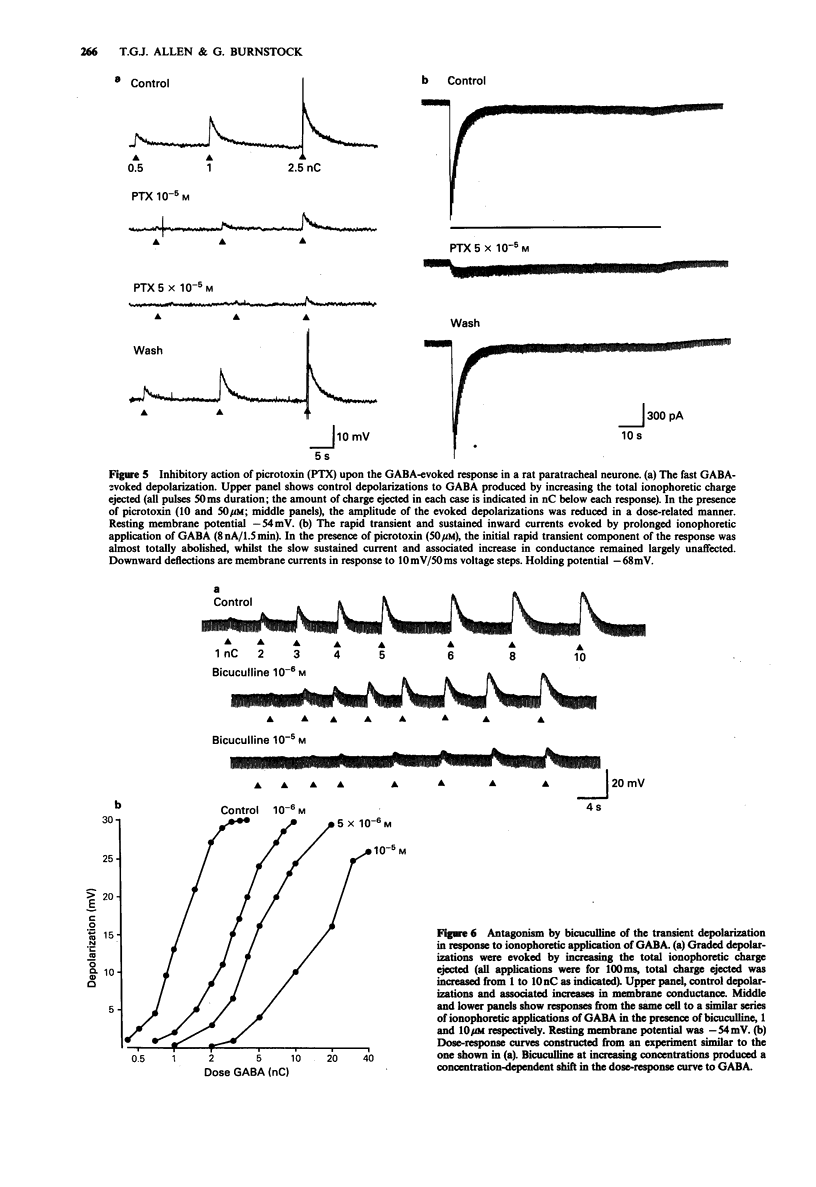
1. The actions of gamma-aminobutyric acid (GABA) on the intramural neurones of 14-18 day old rats were studied in situ by use of intracellular current- and voltage-clamp techniques. The ionic conductance changes and the effects of various GABA-receptor agonists and antagonists on these neurones were also investigated. 2. Prolonged application of GABA either by ionophoresis (10 pC-10 nC) or superfusion (10-100 microM), evoked a biphasic membrane depolarization in over 90% of all paratracheal neurones studied. Typically, the response consisted of an initial rapid depolarization (18-45 ms) that subsequently faded over a period of 15-25 s to reveal a second smaller depolarization which was maintained for the duration of GABA application. Both components of the evoked response resulted in an increase in membrane conductance and an inward flow of current. 3. The amplitude of the transient inward current, recorded during the initial phase of the response, was linearly related to the membrane potential at which it was elicited and reversed symmetrically at a membrane potential of -32.7 mV. The underlying increase in conductance was largely independent of membrane potential. The equilibrium potential for the sustained inward current was -38.7 mV. Replacement of extracellular chloride with gluconate ions initially enhanced the GABA-evoked inward current. With successive applications of GABA in low chloride, the evoked current and conductance changes declined markedly. 4. Muscimol superfusion (1-10 microM) or ionophoresis (10 pC-10 nC) mimicked both the initial and late phases of the GABA-induced conductance change and inward current.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. A voltage-clamp study of the electrophysiological characteristics of the intramural neurones of the rat trachea. J Physiol. 1990 Apr;423:593–614. doi: 10.1113/jphysiol.1990.sp018042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood T. J., Collingridge G. L., Herron C. E., Wheal H. V. Voltage-clamp analysis of somatic gamma-aminobutyric acid responses in adult rat hippocampal CA1 neurones in vitro. J Physiol. 1987 Mar;384:27–37. doi: 10.1113/jphysiol.1987.sp016441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvisi M. G., Ichinose M., Barnes P. J. Modulation of non-adrenergic, non-cholinergic neural bronchoconstriction in guinea-pig airways via GABAB-receptors. Br J Pharmacol. 1989 Aug;97(4):1225–1231. doi: 10.1111/j.1476-5381.1989.tb12582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. -Aminobutyric acid uptake by sympathetic ganglia. Nat New Biol. 1972 Jul 19;238(81):89–91. doi: 10.1038/newbio238089a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., North R. A. Actions of gamma-aminobutyric acid on neurones of guinea-pig myenteric plexus. Br J Pharmacol. 1984 May;82(1):93–100. doi: 10.1111/j.1476-5381.1984.tb16445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., North R. A. Inhibition of calcium spikes and transmitter release by gamma-aminobutyric acid in the guinea-pig myenteric plexus. Br J Pharmacol. 1984 May;82(1):101–105. doi: 10.1111/j.1476-5381.1984.tb16446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. gamma-Aminobutyric acid-induced depression of calcium currents of chick sensory neurons. Neurosci Lett. 1985 May 14;56(2):205–210. doi: 10.1016/0304-3940(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Deschenes M., Feltz P., Lamour Y. A model for an estimate in vivo of the ionic basis of presynaptic inhibition: an intracellular analysis of the GABA-induced depolarization in rat dorsal root ganglia. Brain Res. 1976 Dec 24;118(3):486–493. doi: 10.1016/0006-8993(76)90318-8. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R., Johnston D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J Neurophysiol. 1985 Jul;54(1):134–142. doi: 10.1152/jn.1985.54.1.134. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu M. A., Deal E. C., Jr, Norcia M. P., Van Lunteren E., Mitra J., Cherniack N. S. Medullary effects of nicotine and GABA on tracheal smooth muscle tone. Respir Physiol. 1986 Jun;64(3):351–363. doi: 10.1016/0034-5687(86)90128-3. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Alger B. E. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986 Jul;56(1):1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Hills J. M., Saffrey M. J. Immunohistochemical demonstration of GABAergic neurons in the enteric nervous system. J Neurosci. 1986 Jun;6(6):1628–1634. doi: 10.1523/JNEUROSCI.06-06-01628.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R., Dennison M. E., Burnstock G. GABA may be a neurotransmitter in the vertebrate peripheral nervous system. Nature. 1979 Sep 6;281(5726):71–74. doi: 10.1038/281071a0. [DOI] [PubMed] [Google Scholar]
- Kato E., Kuba K. Inhibition of transmitter release in bullfrog sympathetic ganglia induced by gamma-aminobutyric acid. J Physiol. 1980 Jan;298:271–283. doi: 10.1113/jphysiol.1980.sp013080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato E., Morita K., Kuba K., Yamada S., Kuhara T., Shinka T., Matsumoto I. Does gamma-aminobutyric acid in blood control transmitter release in bullfrog sympathetic ganglia? Brain Res. 1980 Aug 11;195(1):208–214. doi: 10.1016/0006-8993(80)90879-3. [DOI] [PubMed] [Google Scholar]
- Luzzi S., Franchi-Micheli S., Folco G., Rossoni G., Ciuffi M., Zilletti L. Effect of baclofen on different models of bronchial hyperreactivity in the guinea-pig. Agents Actions. 1987 Apr;20(3-4):307–309. doi: 10.1007/BF02074698. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Higashi H., Gallagher J. P., Shinnick-Gallagher P. On the mechanism of action of GABA in pelvic vesical ganglia: biphasic responses evoked by two opposing actions on membrane conductance. Brain Res. 1983 Feb 7;260(2):233–248. doi: 10.1016/0006-8993(83)90677-7. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Higashi H., Shinnick-Gallagher P., Gallagher J. P. A hyperpolarizing GABA response associated with a conductance decrease. Brain Res. 1981 Oct 5;222(1):204–208. doi: 10.1016/0006-8993(81)90960-4. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A., Baker D. G., Basbaum C. B. In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Res. 1987 Dec 22;437(1):157–160. doi: 10.1016/0006-8993(87)91537-x. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Lambert J. J., Cottrell G. A. An electrophysiological investigation of the characteristics and function of GABAA receptors on bovine adrenomedullary chromaffin cells. Pflugers Arch. 1989 Oct;415(1):95–103. doi: 10.1007/BF00373146. [DOI] [PubMed] [Google Scholar]
- Tamaoki J., Graf P. D., Nadel J. A. Effect of gamma-aminobutyric acid on neurally mediated contraction of guinea pig trachealis smooth muscle. J Pharmacol Exp Ther. 1987 Oct;243(1):86–90. [PubMed] [Google Scholar]
- Yasui S., Ishizuka S., Akaike N. GABA activates different types of chloride-conducting receptor-ionophore complexes in a dose-dependent manner. Brain Res. 1985 Sep 30;344(1):176–180. doi: 10.1016/0006-8993(85)91206-5. [DOI] [PubMed] [Google Scholar]
- de Groat W. C. The actions of gamma-aminobutyric acid and related amino acids on mammalian autonomic ganglia. J Pharmacol Exp Ther. 1970 Apr;172(2):384–396. [PubMed] [Google Scholar]


