Abstract
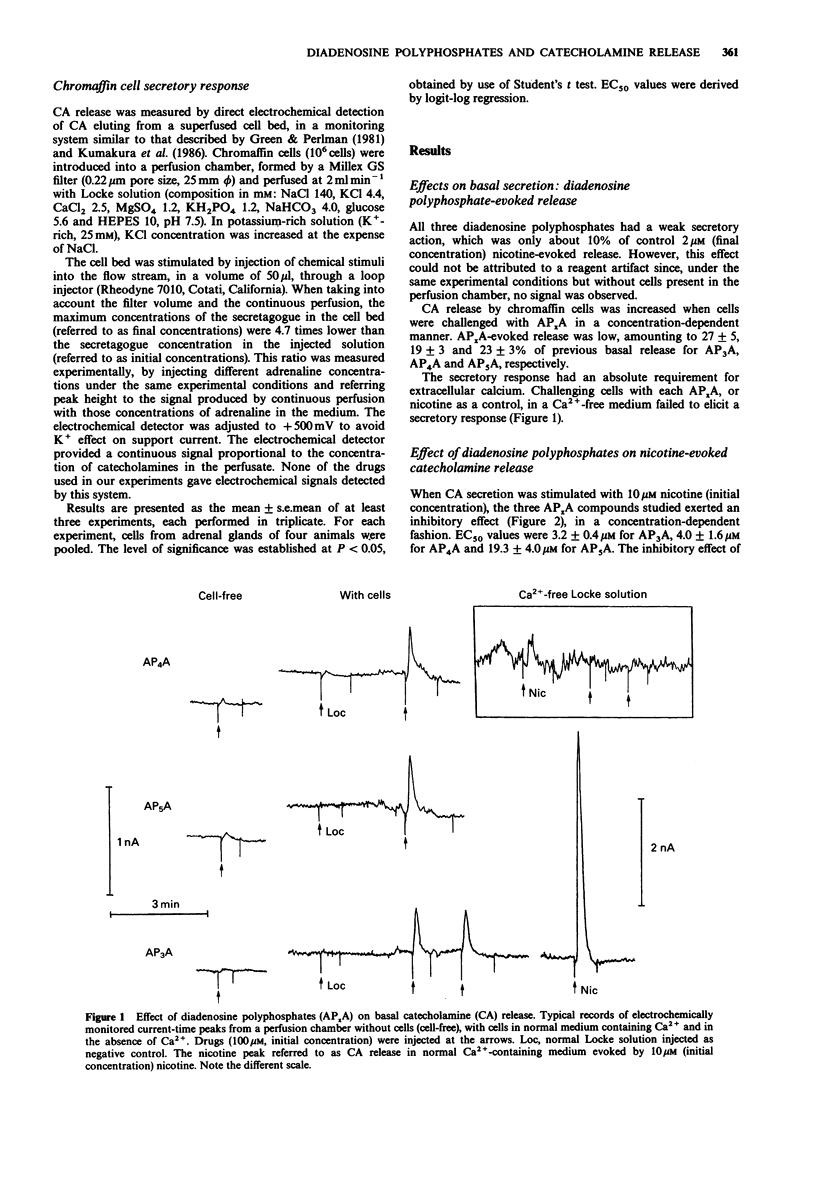
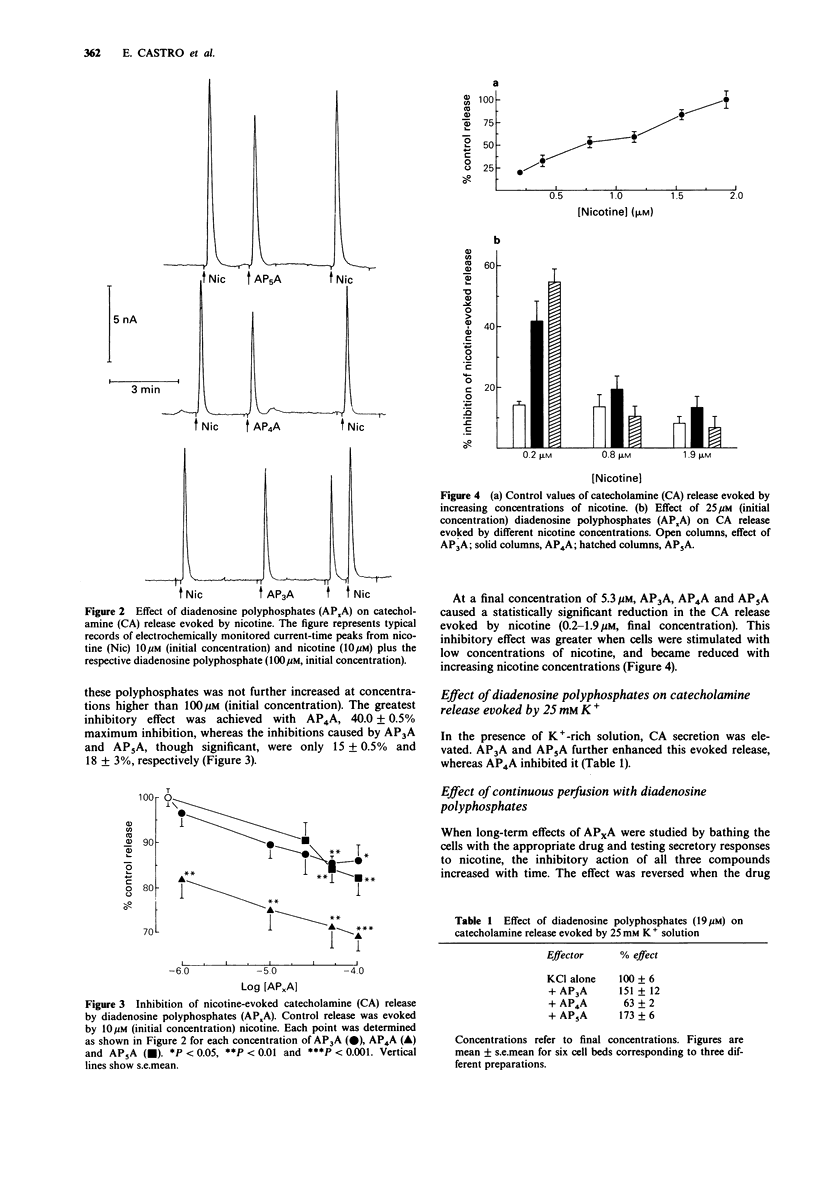
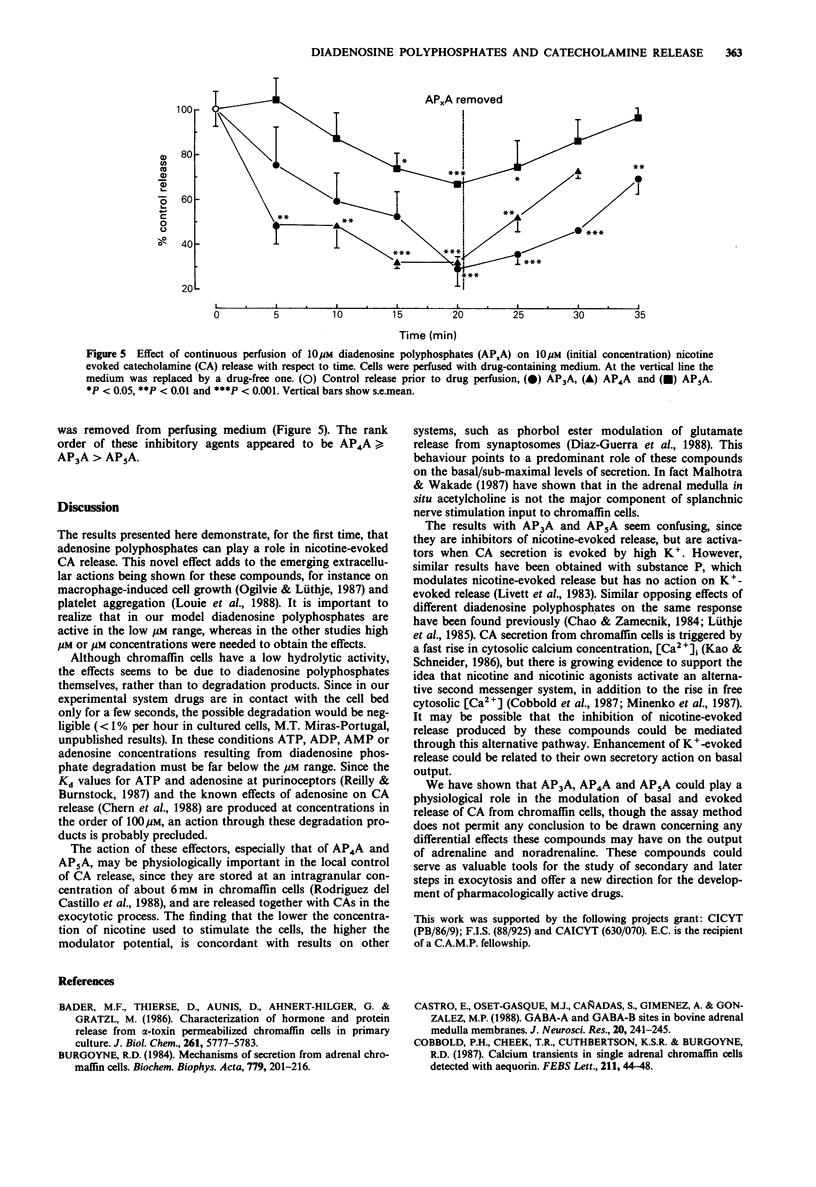
1. The action of several diadenosine polyphosphates (AP3A, AP4A and AP5A) on basal, and on nicotine- and high K(+)-evoked, catecholamine (CA) release has been investigated. Each of the three diadenosine polyphosphates weakly but significantly increased basal CA secretion. This enhancement represented about 10% of the response evoked by 2 microM nicotine. 2. The evoked secretory response to diadenosine polyphosphates had an absolute requirement for extracellular Ca2+. 3. In contrast, these compounds had an inhibitory action on nicotine-evoked release. This response was concentration-dependent, EC50 values being 3.2 +/- 0.4 microM, 4.0 +/- 1.6 microM and 19.3 +/- 4.0 microM for AP3A, AP4A, and AP5A, respectively. The lower the concentration of nicotine used to evoke secretion, the higher the inhibitory power of these compounds. 4. The CA secretion evoked by K(+)-rich solutions was further enhanced by AP3A and AP5A, whereas AP4A inhibited it. The possible physiological role of these dual actions is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Burgoyne R. D. Mechanisms of secretion from adrenal chromaffin cells. Biochim Biophys Acta. 1984 Jun 25;779(2):201–216. doi: 10.1016/0304-4157(84)90009-1. [DOI] [PubMed] [Google Scholar]
- Castro E., Oset-Gasque M. J., Cañadas S., Gimenez G., González M. P. GABAA and GABAB sites in bovine adrenal medulla membranes. J Neurosci Res. 1988;20(2):241–245. doi: 10.1002/jnr.490200213. [DOI] [PubMed] [Google Scholar]
- Chern Y. J., Herrera M., Kao L. S., Westhead E. W. Inhibition of catecholamine secretion from bovine chromaffin cells by adenine nucleotides and adenosine. J Neurochem. 1987 May;48(5):1573–1576. doi: 10.1111/j.1471-4159.1987.tb05703.x. [DOI] [PubMed] [Google Scholar]
- Chern Y. J., Kim K. T., Slakey L. L., Westhead E. W. Adenosine receptors activate adenylate cyclase and enhance secretion from bovine adrenal chromaffin cells in the presence of forskolin. J Neurochem. 1988 May;50(5):1484–1493. doi: 10.1111/j.1471-4159.1988.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Cheek T. R., Cuthbertson K. S., Burgoyne R. D. Calcium transients in single adrenal chromaffin cells detected with aequorin. FEBS Lett. 1987 Jan 19;211(1):44–48. doi: 10.1016/0014-5793(87)81271-1. [DOI] [PubMed] [Google Scholar]
- Díaz-Guerra M. J., Sánchez-Prieto J., Bosca L., Pocock J., Barrie A., Nicholls D. Phorbol ester translocation of protein kinase C in guinea-pig synaptosomes and the potentiation of calcium-dependent glutamate release. Biochim Biophys Acta. 1988 Jun 30;970(2):157–165. doi: 10.1016/0167-4889(88)90174-7. [DOI] [PubMed] [Google Scholar]
- Flodgaard H., Klenow H. Abundant amounts of diadenosine 5',5"'-P1,P4-tetraphosphate are present and releasable, but metabolically inactive, in human platelets. Biochem J. 1982 Dec 15;208(3):737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. J., Perlman R. L. On-line measurement of catecholamine secretion. Anal Biochem. 1981 Jan 15;110(2):270–276. doi: 10.1016/0003-2697(81)90191-3. [DOI] [PubMed] [Google Scholar]
- Greenberg A., Zinder O. Alpha- and beta-receptor control of catecholamine secretion from isolated adrenal medulla cells. Cell Tissue Res. 1982;226(3):655–665. doi: 10.1007/BF00214792. [DOI] [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Calcium mobilization and catecholamine secretion in adrenal chromaffin cells. A Quin-2 fluorescence study. J Biol Chem. 1986 Apr 15;261(11):4881–4888. [PubMed] [Google Scholar]
- Kumakura K., Karoum F., Guidotti A., Costa E. Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature. 1980 Jan 31;283(5746):489–492. doi: 10.1038/283489a0. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Ohara M., Satô G. P. Real-time monitoring of the secretory function of cultured adrenal chromaffin cells. J Neurochem. 1986 Jun;46(6):1851–1858. doi: 10.1111/j.1471-4159.1986.tb08504.x. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Boksa P., Dean D. M., Mizobe F., Lindenbaum M. H. Use of isolated chromaffin cells to study basic release mechanisms. J Auton Nerv Syst. 1983 Jan;7(1):59–86. doi: 10.1016/0165-1838(83)90069-3. [DOI] [PubMed] [Google Scholar]
- Louie S., Kim B. K., Zamecnik P. Diadenosine 5',5'''-P1,P4-tetraphosphate, a potential antithrombotic agent. Thromb Res. 1988 Mar 15;49(6):557–565. doi: 10.1016/0049-3848(88)90253-8. [DOI] [PubMed] [Google Scholar]
- Lüthje J., Baringer J., Ogilvie A. Effects of diadenosine triphosphate (Ap3A) and diadenosine tetraphosphate (Ap4A) on platelet aggregation in unfractionated human blood. Blut. 1985 Dec;51(6):405–413. doi: 10.1007/BF00320727. [DOI] [PubMed] [Google Scholar]
- Lüthje J., Ogilvie A. Catabolism of Ap4A and Ap3A in human serum. Identification of isoenzymes and their partial characterization. Eur J Biochem. 1987 Dec 1;169(2):385–388. doi: 10.1111/j.1432-1033.1987.tb13624.x. [DOI] [PubMed] [Google Scholar]
- Lüthje J., Ogilvie A. Catabolism of Ap4A and Ap3A in whole blood. The dinucleotides are long-lived signal molecules in the blood ending up as intracellular ATP in the erythrocytes. Eur J Biochem. 1988 Apr 5;173(1):241–245. doi: 10.1111/j.1432-1033.1988.tb13990.x. [DOI] [PubMed] [Google Scholar]
- Lüthje J., Ogilvie A. The presence of diadenosine 5',5'''-P1,P3-triphosphate (Ap3A) in human platelets. Biochem Biophys Res Commun. 1983 Aug 30;115(1):253–260. doi: 10.1016/0006-291x(83)90997-x. [DOI] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade A. R. Non-cholinergic component of rat splanchnic nerves predominates at low neuronal activity and is eliminated by naloxone. J Physiol. 1987 Feb;383:639–652. doi: 10.1113/jphysiol.1987.sp016434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minenko A., Kiselev G., Tulkova E., Oehme P. Nicotinic stimulation of polyphosphoinositide turnover in rat adrenal medulla slices. Pharmazie. 1987 May;42(5):341–344. [PubMed] [Google Scholar]
- Miras-Portugal M. T., Torres M., Rotllan P., Aunis D. Adenosine transport in bovine chromaffin cells in culture. J Biol Chem. 1986 Feb 5;261(4):1712–1719. [PubMed] [Google Scholar]
- Mizobe F., Kozousek V., Dean D. M., Livett B. G. Pharmacological characterization of adrenal paraneurons: substance P and somatostatin as inhibitory modulators of the nicotinic response. Brain Res. 1979 Dec 14;178(2-3):555–566. doi: 10.1016/0006-8993(79)90714-5. [DOI] [PubMed] [Google Scholar]
- Newby A. C. The pigeon heart 5'-nucleotidase responsible for ischaemia-induced adenosine formation. Biochem J. 1988 Jul 1;253(1):123–130. doi: 10.1042/bj2530123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly W. M., Burnstock G. The effect of ATP analogues on the spontaneous electrical and mechanical activity of rat portal vein longitudinal muscle. Eur J Pharmacol. 1987 Jun 26;138(3):319–325. doi: 10.1016/0014-2999(87)90469-9. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Brown S. J., Bailyes E. M., Luzio J. P. Ectoenzymes control adenosine modulation of immunoisolated cholinergic synapses. Nature. 1987 May 21;327(6119):232–234. doi: 10.1038/327232a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez del Castillo A., Torres M., Delicado E. G., Miras-Portugal M. T. Subcellular distribution studies of diadenosine polyphosphates--Ap4A and Ap5A--in bovine adrenal medulla: presence in chromaffin granules. J Neurochem. 1988 Dec;51(6):1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Simon J. P., Bader M. F., Aunis D. Secretion from chromaffin cells is controlled by chromogranin A-derived peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1712–1716. doi: 10.1073/pnas.85.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Delicado E. G., Miras-Portugal M. T. Adenosine transporters in chromaffin cells: subcellular distribution and characterization. Biochim Biophys Acta. 1988 Apr 25;969(2):111–120. doi: 10.1016/0167-4889(88)90066-3. [DOI] [PubMed] [Google Scholar]
- Torres M., Molina P., Miras-Portugal M. T. Adenosine transporters in chromaffin cells. Quantification by dipyridamol monoacetate. FEBS Lett. 1986 May 26;201(1):124–128. doi: 10.1016/0014-5793(86)80583-x. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. Diadenosine 5',5"'-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal Biochem. 1983 Oct 1;134(1):1–10. doi: 10.1016/0003-2697(83)90255-5. [DOI] [PubMed] [Google Scholar]


