Abstract
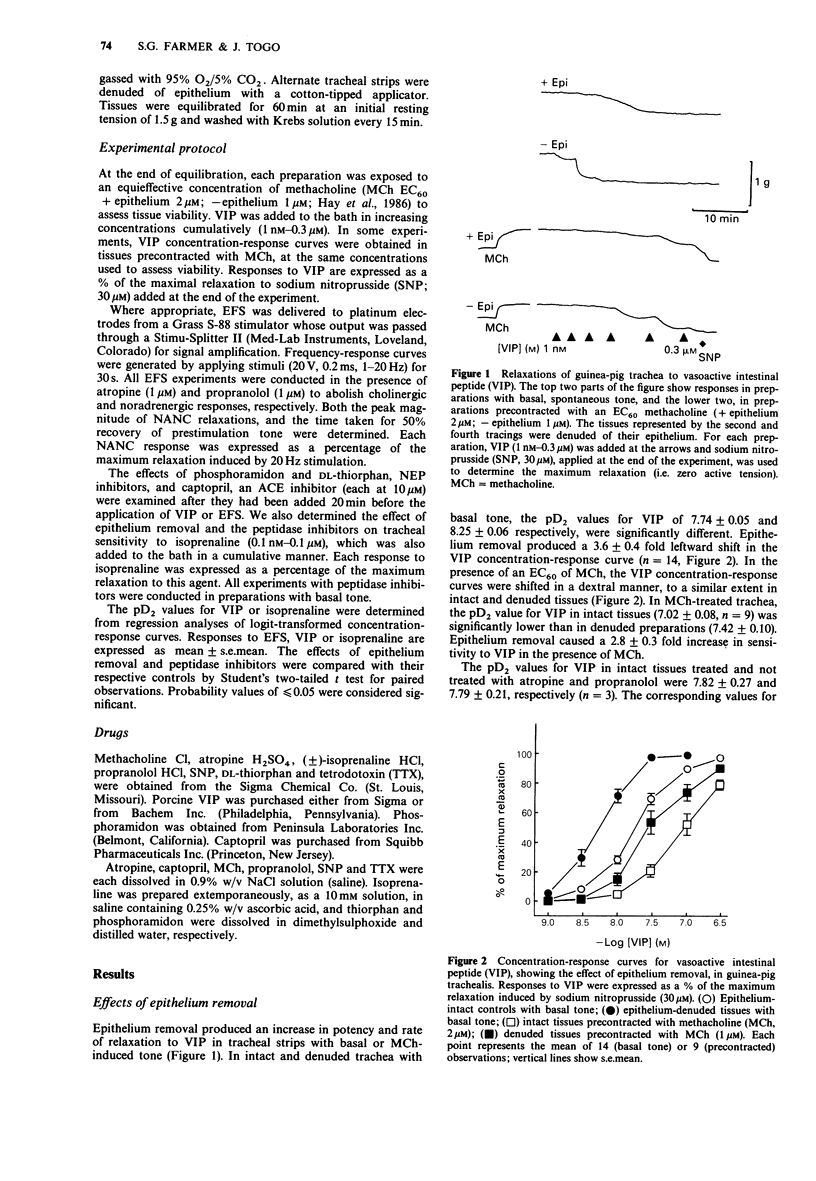
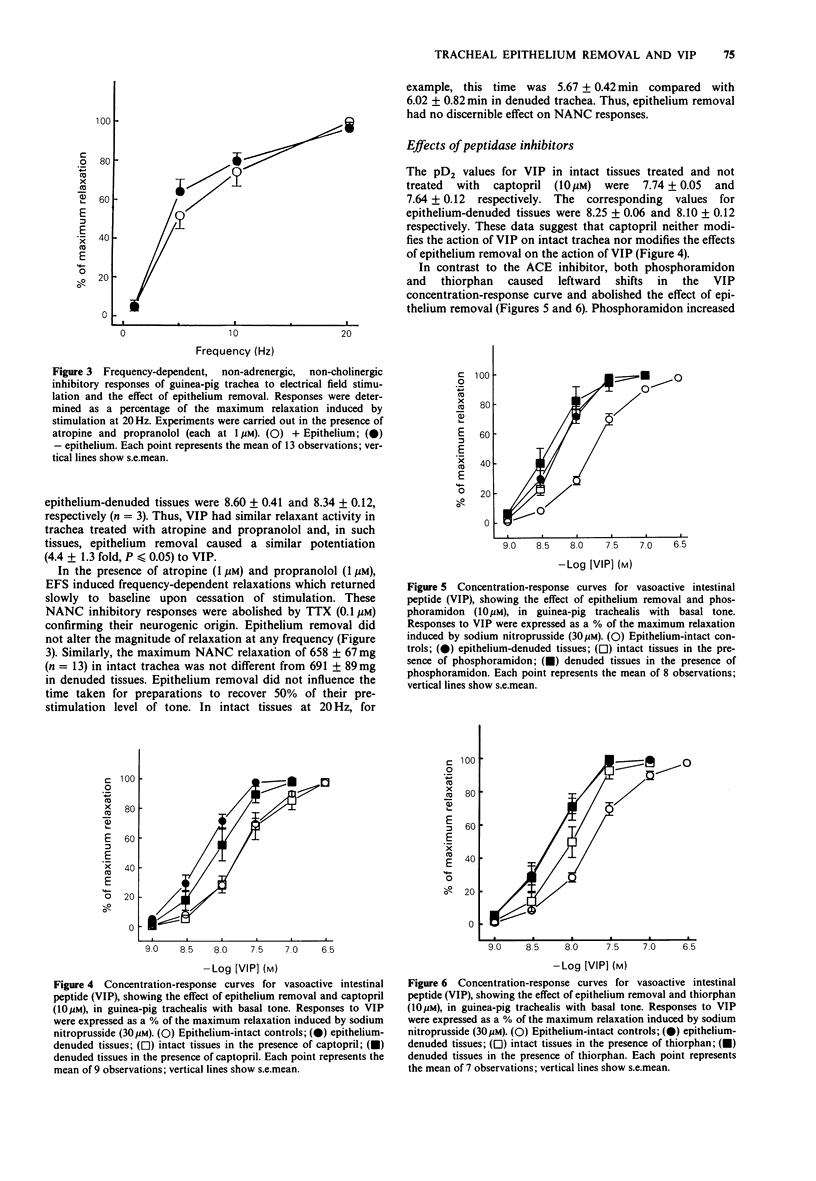
1. We have studied the effect of epithelium removal on relaxation of guinea-pig isolated tracheal smooth muscle induced by vasoactive intestinal peptide (VIP) or stimulation of non-adrenergic, non-cholinergic (NANC) inhibitory nerves. Also examined were the effects of inhibitors of neutral endopeptidase (NEP) and angiotensin-converting enzyme (ACE). 2. Epithelium removal produced a 3.6 +/- 0.4 fold leftward shift in the VIP concentration-response curve. The supersensitivity to VIP, following epithelium removal was abolished by phosphoramidon or thiorphan (NEP inhibitors), but unaffected by captopril (an ACE inhibitor). In intact trachea, the NEP inhibitors produced leftward shifts in the VIP curves similar to those produced by epithelium removal. 3. In contrast to responses to exogenous VIP, neurogenic NANC inhibitory responses to electrical field stimulation were affected neither by epithelial denudation nor by the peptidase inhibitors. 4. As in previous studies, epithelium removal increased tracheal sensitivity to isoprenaline. This was not altered by pretreatment with a cocktail of peptidase inhibitors. Thus, the effect of the NEP inhibitors on responses to VIP appears to be relatively specific. 5. These data indicate that exogenous VIP is a substrate for airway NEP, since inhibition of the enzyme potentiates the peptide. This is further evidence that the airway epithelium provides a source for the metabolism of mediators. 6. In guinea-pig trachea the NEP responsible for cleaving VIP may be located largely in the epithelial layer, since NEP inhibition was without effect on sensitivity to VIP in epithelium-denuded preparations. If VIP is a NANC inhibitory neurotransmitter in this tissue its degradation endogenously does not appear to involve epithelial NEP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Advenier C., Devillier P., Matran R., Naline E. Influence of epithelium on the responsiveness of guinea-pig isolated trachea to adenosine. Br J Pharmacol. 1988 Feb;93(2):295–302. doi: 10.1111/j.1476-5381.1988.tb11434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altiere R. J., Diamond L. Effect of alpha-chymotrypsin on the nonadrenergic noncholinergic inhibitory system in cat airways. Eur J Pharmacol. 1985 Aug 7;114(1):75–78. doi: 10.1016/0014-2999(85)90523-0. [DOI] [PubMed] [Google Scholar]
- Altiere R. J., Diamond L. Relaxation of cat tracheobronchial and pulmonary arterial smooth muscle by vasoactive intestinal peptide: lack of influence by peptidase inhibitors. Br J Pharmacol. 1984 Jun;82(2):321–328. doi: 10.1111/j.1476-5381.1984.tb10766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. Neural control of human airways in health and disease. Am Rev Respir Dis. 1986 Dec;134(6):1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- Barrowcliffe M. P., Morice A., Jones J. G., Sever P. S. Pulmonary clearance of vasoactive intestinal peptide. Thorax. 1986 Feb;41(2):88–93. doi: 10.1136/thx.41.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodanszky M., Klausner Y. S., Said S. I. Biological activities of synthetic peptides corresponding to fragments of and to the entire sequence of the vasoactive intestinal peptide. Proc Natl Acad Sci U S A. 1973 Feb;70(2):382–384. doi: 10.1073/pnas.70.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstairs J. R., Barnes P. J. Visualization of vasoactive intestinal peptide receptors in human and guinea pig lung. J Pharmacol Exp Ther. 1986 Oct;239(1):249–255. [PubMed] [Google Scholar]
- Caughey G. H., Leidig F., Viro N. F., Nadel J. A. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther. 1988 Jan;244(1):133–137. [PubMed] [Google Scholar]
- Devillier P., Advenier C., Drapeau G., Marsac J., Regoli D. Comparison of the effects of epithelium removal and of an enkephalinase inhibitor on the neurokinin-induced contractions of guinea-pig isolated trachea. Br J Pharmacol. 1988 Jul;94(3):675–684. doi: 10.1111/j.1476-5381.1988.tb11575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey R. D., Shannon W. A., Jr, Said S. I. Localization of VIP-immunoreactive nerves in airways and pulmonary vessels of dogs, cat, and human subjects. Cell Tissue Res. 1981;220(2):231–238. doi: 10.1007/BF00210505. [DOI] [PubMed] [Google Scholar]
- Diamond L., Altiere R. J., Thompson D. C. The airway nonadrenergic noncholinergic inhibitory nervous system. Chest. 1988 Jun;93(6):1283–1285. doi: 10.1378/chest.93.6.1283. [DOI] [PubMed] [Google Scholar]
- Diamond L., Szarek J. L., Gillespie M. N., Altiere R. J. In vivo bronchodilator activity of vasoactive intestinal peptide in the cat. Am Rev Respir Dis. 1983 Nov;128(5):827–832. doi: 10.1164/arrd.1983.128.5.827. [DOI] [PubMed] [Google Scholar]
- Djokic T. D., Dusser D. J., Borson D. B., Nadel J. A. Neutral endopeptidase modulates neurotensin-induced airway contraction. J Appl Physiol (1985) 1989 May;66(5):2338–2343. doi: 10.1152/jappl.1989.66.5.2338. [DOI] [PubMed] [Google Scholar]
- Dusser D. J., Djokic T. D., Borson D. B., Nadel J. A. Cigarette smoke induces bronchoconstrictor hyperresponsiveness to substance P and inactivates airway neutral endopeptidase in the guinea pig. Possible role of free radicals. J Clin Invest. 1989 Sep;84(3):900–906. doi: 10.1172/JCI114251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Farmer S. G. Effects of peptidases on non-adrenergic, non-cholinergic inhibitory responses of tracheal smooth muscle: a comparison with effects on VIP- and PHI-induced relaxation. Br J Pharmacol. 1989 Mar;96(3):521–526. doi: 10.1111/j.1476-5381.1989.tb11848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Farmer S. G. Modulation of cholinergic neurotransmission by vasoactive intestinal peptide and peptide histidine isoleucine in guinea-pig tracheal smooth muscle. Pulm Pharmacol. 1989;2(2):107–112. doi: 10.1016/0952-0600(89)90032-x. [DOI] [PubMed] [Google Scholar]
- Ellis J. L., Farmer S. G. The effects of vasoactive intestinal peptide (VIP) antagonists, and VIP and peptide histidine isoleucine antisera on non-adrenergic, non-cholinergic relaxations of tracheal smooth muscle. Br J Pharmacol. 1989 Mar;96(3):513–520. doi: 10.1111/j.1476-5381.1989.tb11847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989 Feb;3(2):145–151. [PubMed] [Google Scholar]
- Farmer S. G., Fedan J. S., Hay D. W., Raeburn D. The effects of epithelium removal on the sensitivity of guinea-pig isolated trachealis to bronchodilator drugs. Br J Pharmacol. 1986 Oct;89(2):407–414. doi: 10.1111/j.1476-5381.1986.tb10274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. G., Hay D. W., Raeburn D., Fedan J. S. Relaxation of guinea-pig tracheal smooth muscle to arachidonate is converted to contraction following epithelium removal. Br J Pharmacol. 1987 Sep;92(1):231–236. doi: 10.1111/j.1476-5381.1987.tb11316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. G., Togo J. Epithelium removal increases airway smooth muscle sensitivity to vasoactive intestinal peptide: effects of peptidase inhibitors. Br J Pharmacol. 1989 Dec;98 (Suppl):784P–784P. [PubMed] [Google Scholar]
- Fernandes L. B., Paterson J. W., Goldie R. G. Co-axial bioassay of a smooth muscle relaxant factor released from guinea-pig tracheal epithelium. Br J Pharmacol. 1989 Jan;96(1):117–124. doi: 10.1111/j.1476-5381.1989.tb11791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J. M., Gordon T., Sheppard D. Epithelium removal alters responsiveness of guinea pig trachea to substance P. J Appl Physiol (1985) 1989 Jan;66(1):232–237. doi: 10.1152/jappl.1989.66.1.232. [DOI] [PubMed] [Google Scholar]
- Franconi G. M., Graf P. D., Lazarus S. C., Nadel J. A., Caughey G. H. Mast cell tryptase and chymase reverse airway smooth muscle relaxation induced by vasoactive intestinal peptide in the ferret. J Pharmacol Exp Ther. 1989 Mar;248(3):947–951. [PubMed] [Google Scholar]
- Frossard N., Rhoden K. J., Barnes P. J. Influence of epithelium on guinea pig airway responses to tachykinins: role of endopeptidase and cyclooxygenase. J Pharmacol Exp Ther. 1989 Jan;248(1):292–298. [PubMed] [Google Scholar]
- Gabella G. Innervation of airway smooth muscle: fine structure. Annu Rev Physiol. 1987;49:583–594. doi: 10.1146/annurev.ph.49.030187.003055. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Sreedharan S. P., Turck C. W., Bridenbaugh R., Malfroy B. Preferential cleavage of amino- and carboxyl-terminal oligopeptides from vasoactive intestinal polypeptide by human recombinant enkephalinase (neutral endopeptidase, EC 3.4.24.11). Biochem Biophys Res Commun. 1989 Feb 15;158(3):850–854. doi: 10.1016/0006-291x(89)92800-3. [DOI] [PubMed] [Google Scholar]
- Goldie R. G., Fernandes L. B., Farmer S. G., Hay D. W. Airway epithelium-derived inhibitory factor. Trends Pharmacol Sci. 1990 Feb;11(2):67–70. doi: 10.1016/0165-6147(90)90320-8. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Farmer S. G., Raeburn D., Robinson V. A., Fleming W. W., Fedan J. S. Airway epithelium modulates the reactivity of guinea-pig respiratory smooth muscle. Eur J Pharmacol. 1986 Sep 23;129(1-2):11–18. doi: 10.1016/0014-2999(86)90330-4. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Muccitelli R. M., Horstemeyer D. L., Wilson K. A., Raeburn D. Demonstration of the release of an epithelium-derived inhibitory factor from a novel preparation of guinea-pig trachea. Eur J Pharmacol. 1987 Apr 14;136(2):247–250. doi: 10.1016/0014-2999(87)90719-9. [DOI] [PubMed] [Google Scholar]
- Holroyde M. C. The influence of epithelium on the responsiveness of guinea-pig isolated trachea. Br J Pharmacol. 1986 Mar;87(3):501–507. doi: 10.1111/j.1476-5381.1986.tb10192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Takeda K. Non-adrenergic inhibitory nerves and putative transmitters in the smooth muscle of cat trachea. J Physiol. 1982 Sep;330:497–511. doi: 10.1113/jphysiol.1982.sp014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Ashton J., Schulz W. W., Erdös E. G. Neutral metalloendopeptidase in human lung tissue and cultured cells. Am Rev Respir Dis. 1985 Sep;132(3):564–568. doi: 10.1164/arrd.1985.132.3.564. [DOI] [PubMed] [Google Scholar]
- Laitinen A. Autonomic innervation of the human respiratory tract as revealed by histochemical and ultrastructural methods. Eur J Respir Dis Suppl. 1985;140:1–42. [PubMed] [Google Scholar]
- Lundberg J. M., Fahrenkrug J., Hökfelt T., Martling C. R., Larsson O., Tatemoto K., Anggård A. Co-existence of peptide HI (PHI) and VIP in nerves regulating blood flow and bronchial smooth muscle tone in various mammals including man. Peptides. 1984 May-Jun;5(3):593–606. doi: 10.1016/0196-9781(84)90090-1. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y., Hamasaki Y., Said S. I. Vasoactive intestinal peptide: a possible transmitter of nonadrenergic relaxation of guinea pig airways. Science. 1980 Dec 12;210(4475):1252–1253. doi: 10.1126/science.6254154. [DOI] [PubMed] [Google Scholar]
- Nijkamp F. P., Folkerts G. Reversal of arachidonic acid-induced guinea-pig tracheal relaxation into contraction after epithelium removal. Eur J Pharmacol. 1986 Nov 19;131(2-3):315–316. doi: 10.1016/0014-2999(86)90591-1. [DOI] [PubMed] [Google Scholar]
- Ryan J. W. Peptidase enzymes of the pulmonary vascular surface. Am J Physiol. 1989 Aug;257(2 Pt 1):L53–L60. doi: 10.1152/ajplung.1989.257.2.L53. [DOI] [PubMed] [Google Scholar]
- Said S. I. Vasoactive intestinal peptide in the lung. Ann N Y Acad Sci. 1988;527:450–464. doi: 10.1111/j.1749-6632.1988.tb26999.x. [DOI] [PubMed] [Google Scholar]
- Said S. I. Vasoactive peptides in the lung, with special reference to vasoactive intestinal peptide. Exp Lung Res. 1982 Nov;3(3-4):343–348. doi: 10.3109/01902148209069662. [DOI] [PubMed] [Google Scholar]
- Sundler F., Ekblad E., Grunditz T., Håkanson R., Uddman R. Vasoactive intestinal peptide in the peripheral nervous system. Ann N Y Acad Sci. 1988;527:143–167. doi: 10.1111/j.1749-6632.1988.tb26979.x. [DOI] [PubMed] [Google Scholar]
- Thompson D. C., Altiere R. J., Diamond L. The effects of antagonists of vasoactive intestinal peptide on nonadrenergic noncholinergic inhibitory responses in feline airways. Peptides. 1988 Mar-Apr;9(2):443–447. doi: 10.1016/0196-9781(88)90284-7. [DOI] [PubMed] [Google Scholar]
- Thompson D. C., Wells J. L., Altiere R. J., Diamond L. The effect of epithelium removal on non-adrenergic, non-cholinergic inhibitory responses in the isolated central airways of the cat and guinea pig. Eur J Pharmacol. 1988 Jan 12;145(2):231–237. doi: 10.1016/0014-2999(88)90237-3. [DOI] [PubMed] [Google Scholar]
- Thompson J. E., Sheppard D. Phosphoramidon potentiates the increase in lung resistance mediated by tachykinins in guinea pigs. Am Rev Respir Dis. 1988 Feb;137(2):337–340. doi: 10.1164/ajrccm/137.2.337. [DOI] [PubMed] [Google Scholar]
- Tschirhart E., Schmitt P., Bertrand C., Mayer M., Magneney S., Landry Y., Michelot R. Contractile activity of the N-acylated C-terminal part of substance P7-11 in guinea pig trachea. Effect of epithelium removal. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jul;340(1):107–110. doi: 10.1007/BF00169215. [DOI] [PubMed] [Google Scholar]


