Abstract
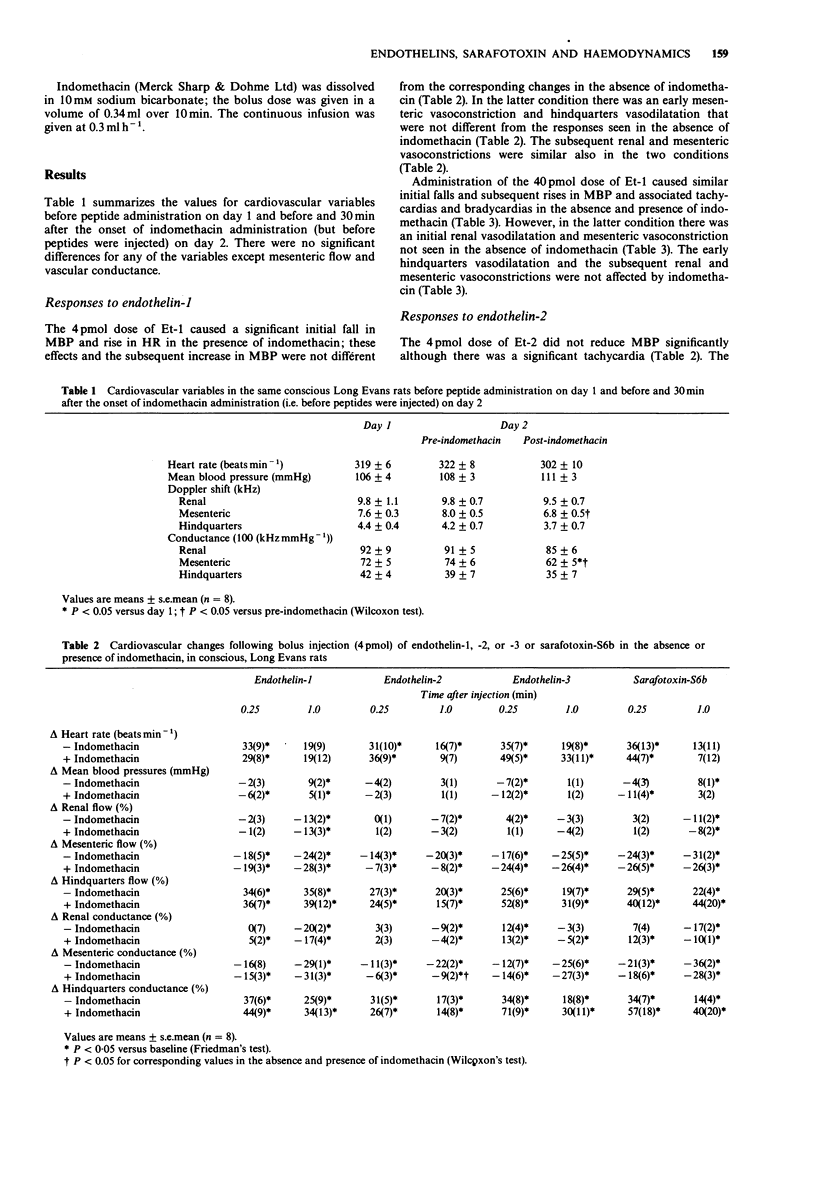
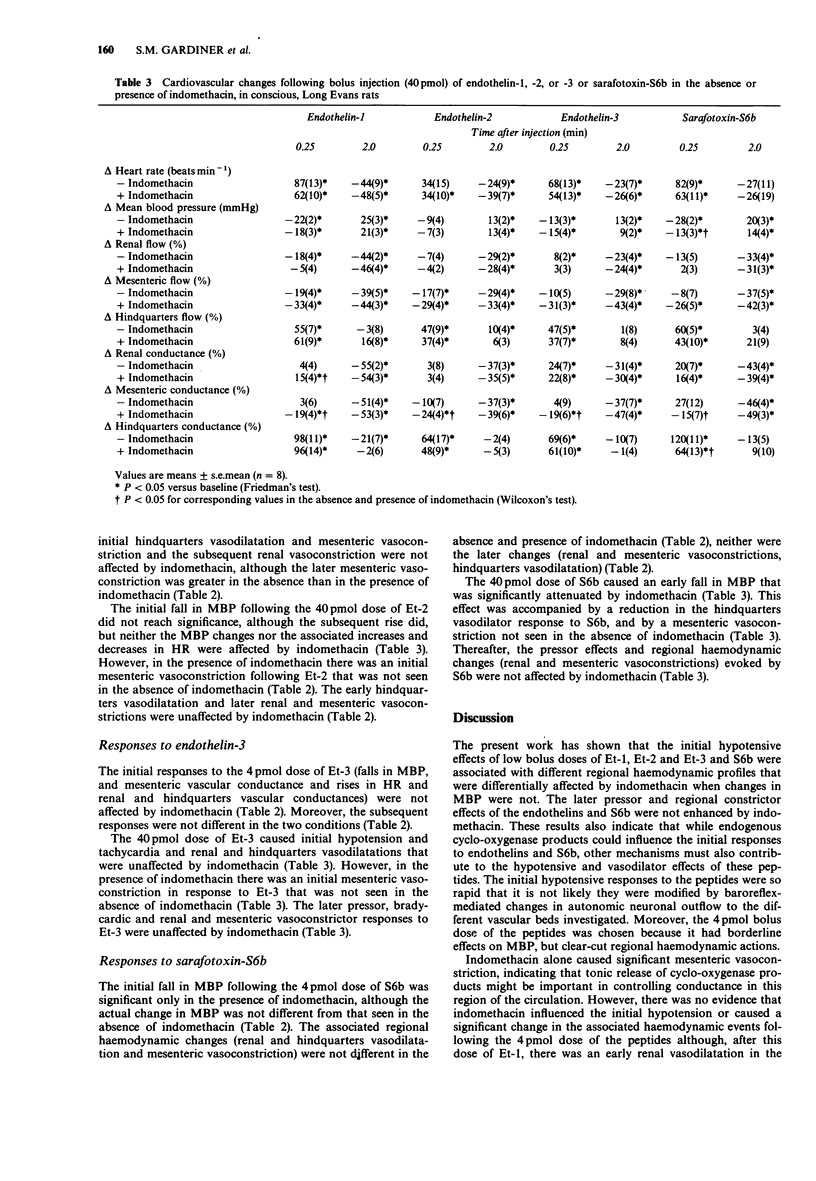
1. Regional haemodynamic responses to i.v. bolus injections of low doses (4 pmol and 40 pmol) of endothelin-1, -2, -3 and sarafotoxin-S6b were assessed in conscious, Long Evans rats in the absence and presence of indomethacin. 2. Both doses of endothelin-3 and sarafotoxin-S6b caused early renal vasodilatations that were not affected by indomethacin. Endothelin-1 caused an initial renal vasodilatation only in the presence of indomethacin, indicating that this peptide produced concurrent release of cyclo-oxygenase products that caused renal vasoconstriction. Neither dose of endothelin-2 produced an increase in renal conductance. 3. The 4 pmol dose of all four peptides caused mesenteric vasoconstrictions only. With the 40 pmol dose of the peptides, none caused early mesenteric vasoconstriction except in the presence of indomethacin. Thus, in this vascular bed the primary vasoconstrictor effects of the peptides (seen with the 4 pmol dose) were offset, following the 40 pmol dose, by release of vasodilator cyclo-oxygenase products. Indomethacin alone caused significant vasoconstriction only in the mesenteric vascular bed, indicating that in this region of the circulation, vasodilator prostanoids might be involved also in the tonic control of vascular conductance. 4. All four peptides at both doses caused early hindquarters vasodilatation. However, only the initial hypotensive and hindquarters vasodilator effects of the 40 pmol dose of sarafotoxin-S6b were attenuated by indomethacin. Under these conditions the hindquarters vasodilator effects of sarafotoxin-S6b were similar to those of the other peptides, indicating that the more marked effects of sarafotoxin-S6b in the absence of indomethacin were contributed to by vasodilator cyclo-oxygenase products in the hindquarters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. NG-monomethyl-L-arginine does not inhibit the hindquarters vasodilator action of endothelin-1 in conscious rats. Eur J Pharmacol. 1989 Nov 21;171(2-3):237–240. doi: 10.1016/0014-2999(89)90113-1. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T. Regional haemodynamic effects of depressor neuropeptides in conscious, unrestrained, Long Evans and Brattleboro rats. Br J Pharmacol. 1988 Sep;95(1):197–208. doi: 10.1111/j.1476-5381.1988.tb16565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J. G., Nies A. S. The hemodynamic effects of prostaglandins in the rat. Evidence for important species variation in renovascular responses. Circ Res. 1979 Mar;44(3):406–410. doi: 10.1161/01.res.44.3.406. [DOI] [PubMed] [Google Scholar]
- Haywood J. R., Shaffer R. A., Fastenow C., Fink G. D., Brody M. J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. Am J Physiol. 1981 Aug;241(2):H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- Hermán F., Magyar K., Chabrier P. E., Braquet P., Filep J. Prostacyclin mediates antiaggregatory and hypotensive actions of endothelin in anaesthetized beagle dogs. Br J Pharmacol. 1989 Sep;98(1):38–40. doi: 10.1111/j.1476-5381.1989.tb16859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog Y., Ambar I., Sokolovsky M., Kochva E., Wollberg Z., Bdolah A. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 1988 Oct 14;242(4876):268–270. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]
- Lidbury P. S., Thiemermann C., Thomas G. R., Vane J. R. Endothelin-3: selectivity as an anti-aggregatory peptide in vivo. Eur J Pharmacol. 1989 Jul 18;166(2):335–338. doi: 10.1016/0014-2999(89)90079-4. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Myers H. A., Honig C. R. Influence of initial resistance on magnitude of response to vasomotor stimuli. Am J Physiol. 1969 Jun;216(6):1429–1436. doi: 10.1152/ajplegacy.1969.216.6.1429. [DOI] [PubMed] [Google Scholar]
- Quilley J., McGiff J. C., Nasjletti A. Role of endoperoxides in arachidonic acid-induced vasoconstriction in the isolated perfused kidney of the rat. Br J Pharmacol. 1989 Jan;96(1):111–116. doi: 10.1111/j.1476-5381.1989.tb11790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae G. A., Trybulec M., de Nucci G., Vane J. R. Endothelin-1 releases eicosanoids from rabbit isolated perfused kidney and spleen. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S89–S102. doi: 10.1097/00005344-198900135-00022. [DOI] [PubMed] [Google Scholar]
- Rakugi H., Nakamaru M., Tabuchi Y., Nagano M., Mikami H., Ogihara T. Endothelin stimulates the release of prostacyclin from rat mesenteric arteries. Biochem Biophys Res Commun. 1989 Apr 28;160(2):924–928. doi: 10.1016/0006-291x(89)92523-0. [DOI] [PubMed] [Google Scholar]
- Similarity of endothelin to snake venom toxin. Nature. 1988 Sep 22;335(6188):303–303. doi: 10.1038/335303a0. [DOI] [PubMed] [Google Scholar]
- Similarity of endothelin to snake venom toxin. Nature. 1988 Sep 22;335(6188):303–303. doi: 10.1038/335303a0. [DOI] [PubMed] [Google Scholar]
- Takasaki C., Tamiya N., Bdolah A., Wollberg Z., Kochva E. Sarafotoxins S6: several isotoxins from Atractaspis engaddensis (burrowing asp) venom that affect the heart. Toxicon. 1988;26(6):543–548. doi: 10.1016/0041-0101(88)90234-6. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Lidbury P. S., Thomas G. R., Vane J. R. Endothelin-1 releases prostacyclin and inhibits ex vivo platelet aggregation in the anesthetized rabbit. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S138–S142. [PubMed] [Google Scholar]
- Walder C. E., Thomas G. R., Thiemermann C., Vane J. R. The hemodynamic effects of endothelin-1 in the pithed rat. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S93–S102. doi: 10.1097/00005344-198900135-00023. [DOI] [PubMed] [Google Scholar]
- Warner T. D., Mitchell J. A., de Nucci G., Vane J. R. Endothelin-1 and endothelin-3 release EDRF from isolated perfused arterial vessels of the rat and rabbit. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S85–S102. doi: 10.1097/00005344-198900135-00021. [DOI] [PubMed] [Google Scholar]
- Warner T. D., de Nucci G., Vane J. R. Rat endothelin is a vasodilator in the isolated perfused mesentery of the rat. Eur J Pharmacol. 1989 Jan 17;159(3):325–326. doi: 10.1016/0014-2999(89)90167-2. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist R. J., Bunting P. B., Garsky V. M., Lumma P. K., Schofield T. L. Prominent depressor response to endothelin in spontaneously hypertensive rats. Eur J Pharmacol. 1989 Apr 12;163(1):199–203. doi: 10.1016/0014-2999(89)90420-2. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]


