Abstract
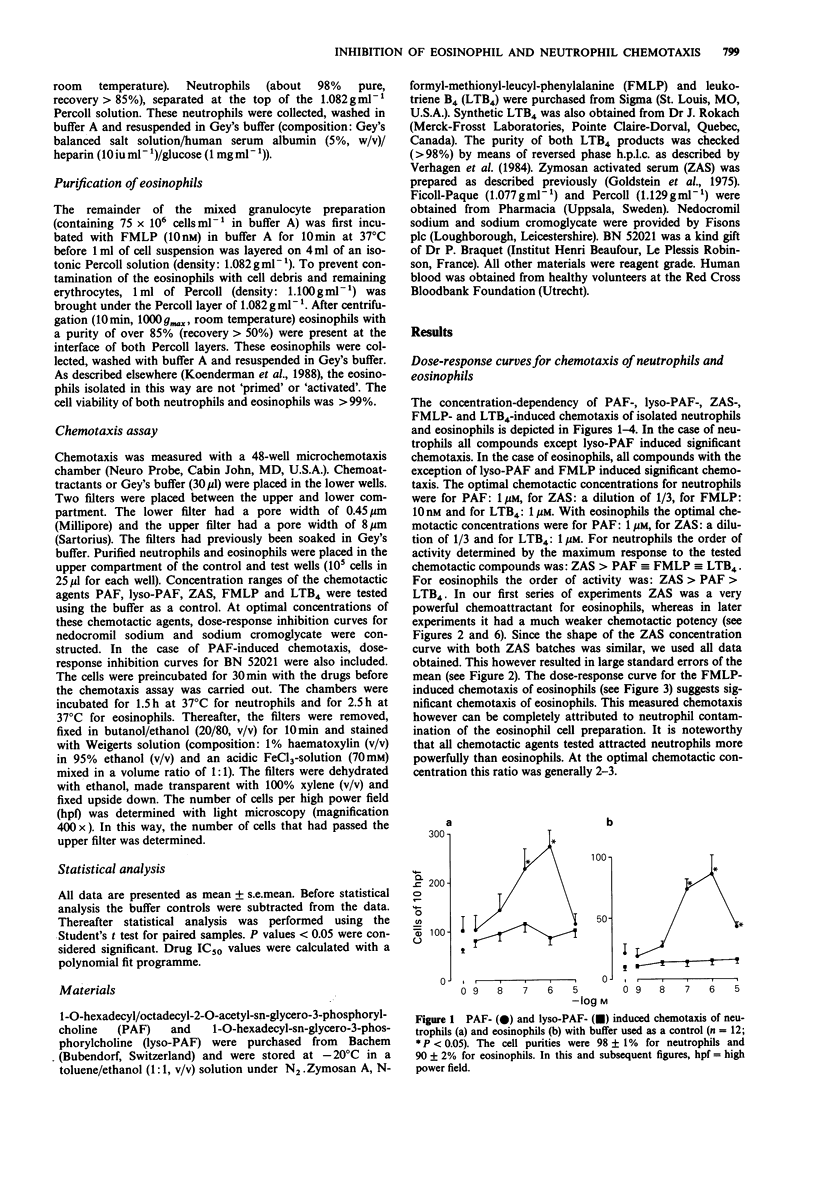
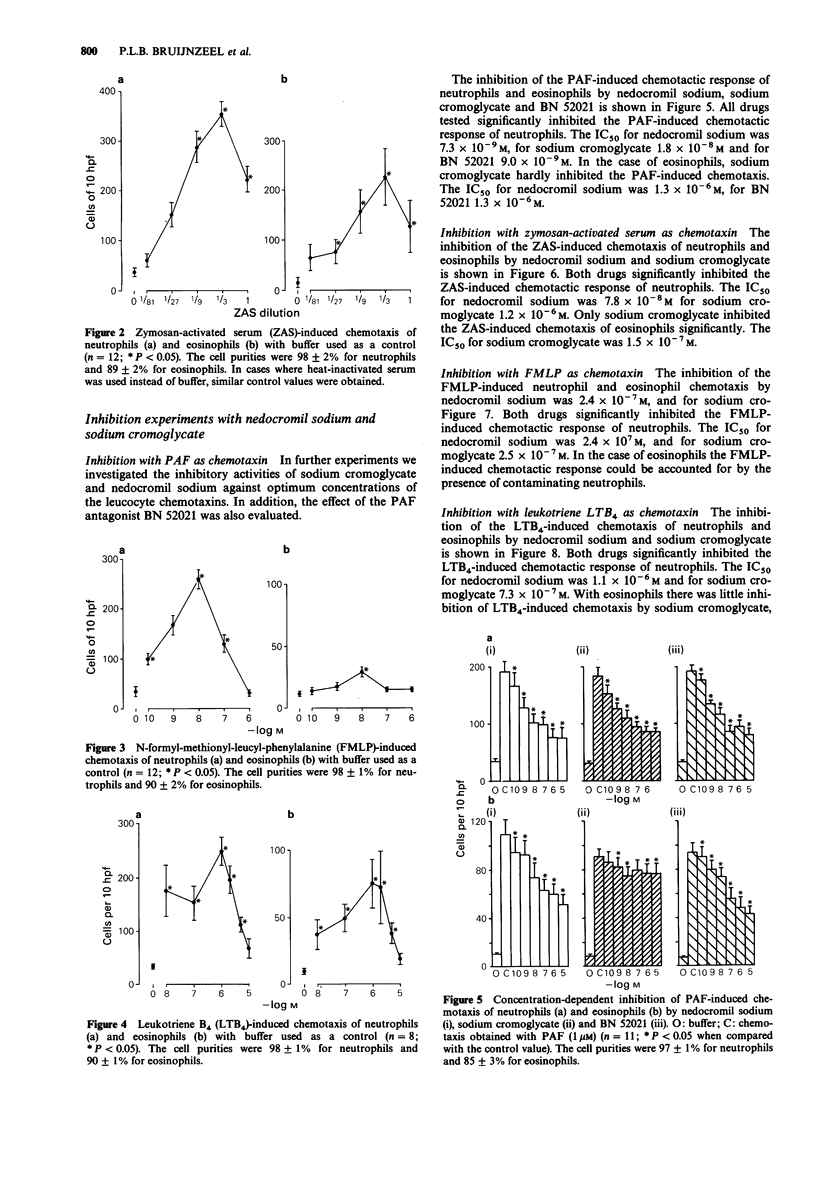
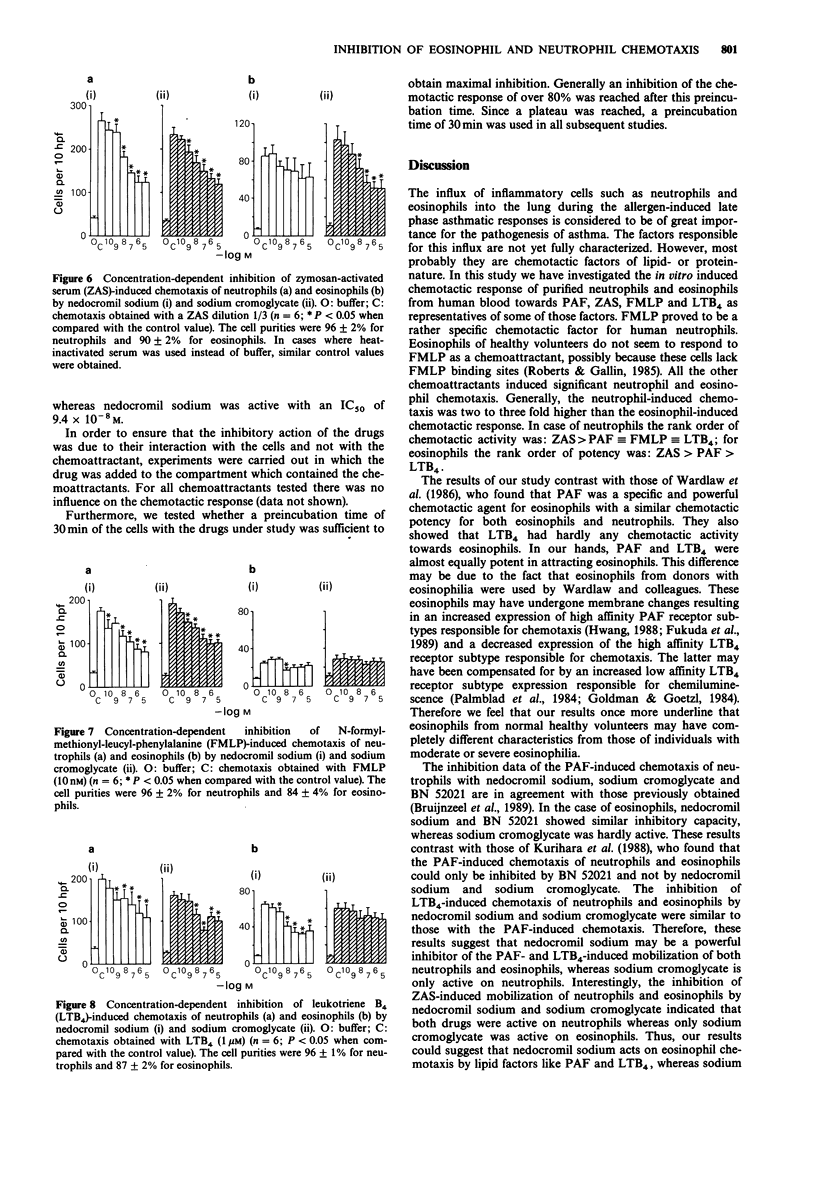
1. Neutrophils and eosinophils infiltrate the airways in association with the allergen-induced late phase asthmatic reaction. Mobilization of these cells takes place via lipid-like and protein-like chemotactic factors. In this study platelet-activating factor (PAF), leukotriene B4 (LTB4), zymosan-activated serum (ZAS) and N-formyl-methionyl-leucyl-phenylalanine (FMLP) were used as illustrative examples of both groups. Chemotaxis was studied in human neutrophils and eosinophils. The inhibitory effects of nedocromil sodium and sodium cromoglycate were evaluated. 2. All chemotactic factors tested attracted neutrophils with the following rank order of activity: ZAS greater than PAF identical to FMLP identical to LTB4. Eosinophils were only mobilized by PAF, LTB4 and ZAS with the following rank order of activity: ZAS greater than PAF greater than LTB4. 3. Nedocromil sodium and sodium cromoglycate were equally active as the PAF antagonist BN 52021 in inhibiting the PAF-induced chemotaxis of neutrophils (IC50 approximately 10(-8) M). Both drugs were also equally active in inhibiting the chemotaxis of neutrophils induced by ZAS (IC50 approximately 10(-7)-10(-6) M), FMLP (IC50 approximately 10(-7) M) and LTB4 (IC50 approximately 10(-6) M). 4. Nedocromil sodium significantly inhibited the chemotaxis of eosinophils induced by PAF (IC50 approximately 10(-6) M) and LTB4 (IC50 approximately 10(-7) M). The inhibitory potency of BN 52021 was similar to that of nedocromil sodium on the PAF-induced chemotaxis of eosinophils. Sodium cromoglycate was incapable of eliciting significant inhibition of these chemotactic responses. However, sodium cromoglycate significantly inhibited the chemotaxis of eosinophils induced by ZAS (IC50 approximately 10(-7) M), whereas nedocromil sodium was ineffective.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins P. C., Wasserman S. I. Chemotactic mediators. Clin Rev Allergy. 1983 Sep;1(3):385–395. doi: 10.1007/BF02991228. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. The differential effects of nedocromil sodium and sodium cromoglycate on the secretory response of rabbit peritoneal neutrophils. Eur J Respir Dis Suppl. 1986;147:238–240. [PubMed] [Google Scholar]
- Bruijnzeel P. L., Warringa R. A., Kok P. T. Inhibition of platelet-activating factor- and zymosan-activated serum-induced chemotaxis of human neutrophils by nedocromil sodium, BN 52021 and sodium cromoglycate. Br J Pharmacol. 1989 Aug;97(4):1251–1257. doi: 10.1111/j.1476-5381.1989.tb12586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monchy J. G., Kauffman H. F., Venge P., Koëter G. H., Jansen H. M., Sluiter H. J., De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985 Mar;131(3):373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Durham S. R., Kay A. B. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy. 1985 Sep;15(5):411–418. doi: 10.1111/j.1365-2222.1985.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Selective transduction of human polymorphonuclear leukocyte functions by subsets of receptors for leukotriene B4. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):373–377. doi: 10.1016/0091-6749(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. B. Identification of a second putative receptor of platelet-activating factor from human polymorphonuclear leukocytes. J Biol Chem. 1988 Mar 5;263(7):3225–3233. [PubMed] [Google Scholar]
- Kay A. B. The mode of action of anti-allergic drugs. Clin Allergy. 1987 Mar;17(2):153–164. [PubMed] [Google Scholar]
- Koenderman L., Kok P. T., Hamelink M. L., Verhoeven A. J., Bruijnzeel P. L. An improved method for the isolation of eosinophilic granulocytes from peripheral blood of normal individuals. J Leukoc Biol. 1988 Aug;44(2):79–86. doi: 10.1002/jlb.44.2.79. [DOI] [PubMed] [Google Scholar]
- Lucas A. M., Shuster S. Cromolyn inhibition of protein kinase C activity. Biochem Pharmacol. 1987 Feb 15;36(4):562–565. doi: 10.1016/0006-2952(87)90368-6. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Hunninghake G. W., Richerson H. B. Late asthmatic responses: inquiry into mechanisms and significance. Clin Rev Allergy. 1985 May;3(2):145–165. doi: 10.1007/BF02992980. [DOI] [PubMed] [Google Scholar]
- Palmblad J., Gyllenhammar H., Lindgren J. A., Malmsten C. L. Effects of leukotrienes and f-Met-Leu-Phe on oxidative metabolism of neutrophils and eosinophils. J Immunol. 1984 Jun;132(6):3041–3045. [PubMed] [Google Scholar]
- Pincus S. H., Schooley W. R., DiNapoli A. M., Broder S. Metabolic heterogeneity of eosinophils from normal and hypereosinophilic patients. Blood. 1981 Dec;58(6):1175–1181. [PubMed] [Google Scholar]
- Prin L., Charon J., Capron M., Gosset P., Taelman H., Tonnel A. B., Capron A. Heterogeneity of human eosinophils. II. Variability of respiratory burst activity related to cell density. Clin Exp Immunol. 1984 Sep;57(3):735–742. [PMC free article] [PubMed] [Google Scholar]
- Roberts R. L., Gallin J. I. Rapid method for isolation of normal human peripheral blood eosinophils on discontinuous Percoll gradients and comparison with neutrophils. Blood. 1985 Feb;65(2):433–440. [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Udén A. M., Palmblad J., Lindgren J. A., Malmsten C. Effects of novel lipoxygenase products on migration of eosinophils and neutrophils in vitro. Int Arch Allergy Appl Immunol. 1983;72(1):91–93. doi: 10.1159/000234847. [DOI] [PubMed] [Google Scholar]
- Verhagen J., Bruynzeel P. L., Koedam J. A., Wassink G. A., de Boer M., Terpstra G. K., Kreukniet J., Veldink G. A., Vliegenthart J. F. Specific leukotriene formation by purified human eosinophils and neutrophils. FEBS Lett. 1984 Mar 12;168(1):23–28. doi: 10.1016/0014-5793(84)80199-4. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Moqbel R., Cromwell O., Kay A. B. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986 Dec;78(6):1701–1706. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]


