Abstract
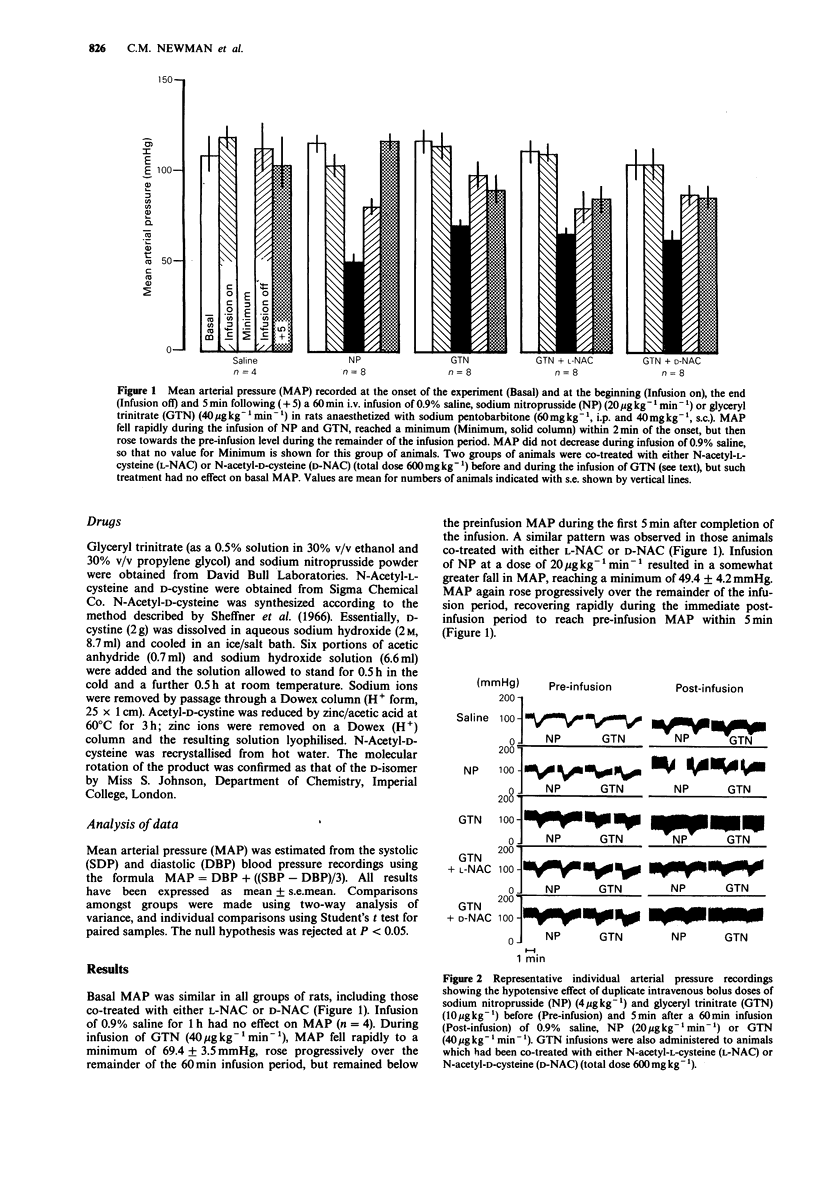
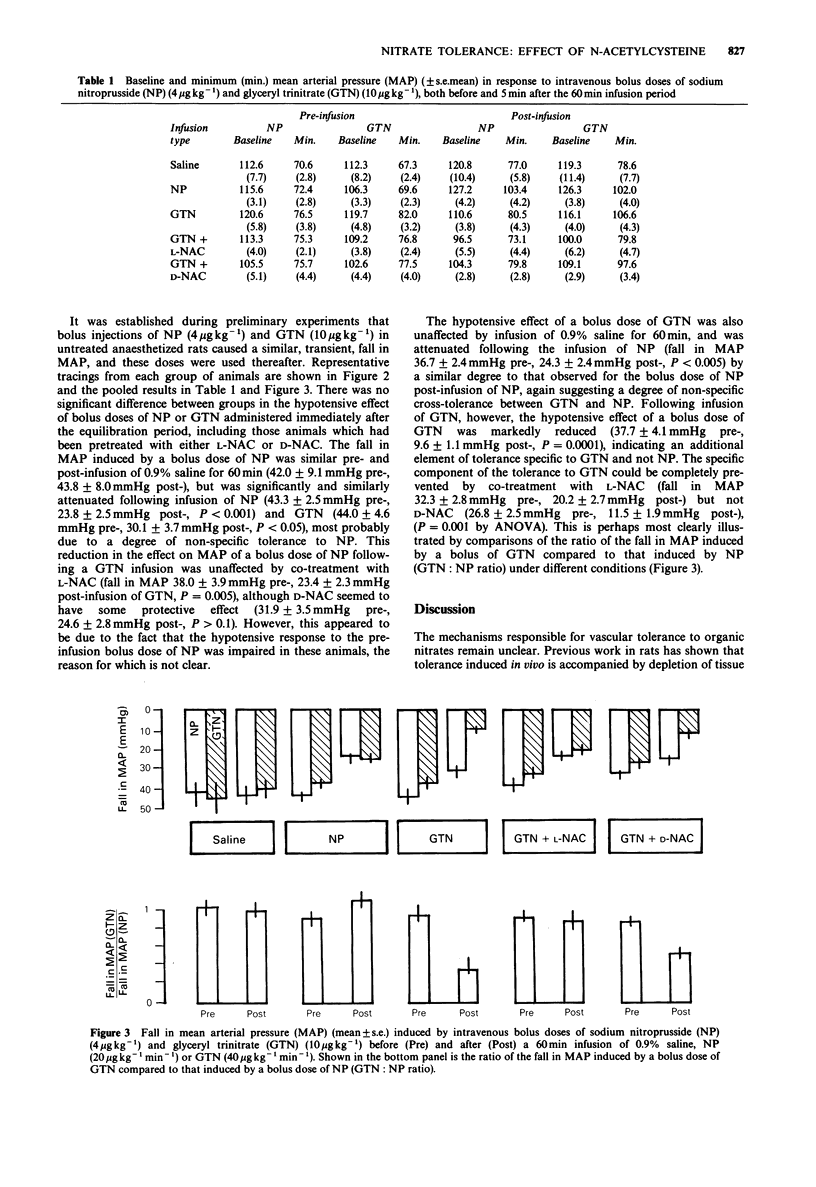
1. A new model of tolerance to the hypotensive effect of organic nitrates has been developed in the rat. 2. The fall in mean arterial pressure (MAP) in response to bolus doses of sodium nitroprusside (NP) (4 micrograms kg-1) and glyceryl trinitrate (GTN) (10 micrograms kg-1) was recorded both before and after a 60 min infusion of either 0.9% saline, NP (20 micrograms kg-1 min-1) or GTN (40 micrograms kg-1 min-1). 3. The hypotensive effects of NP or GTN were unchanged following saline infusion, but were reduced in both cases by approximately 40% following the infusion of NP. 4. Infusion of GTN for 60 min virtually abolished the hypotensive effect of a GTN bolus (i.e. nitrate tolerance), whilst the effect of a NP bolus was reduced only to a similar extent (30%) as after an infusion of NP. This latter effect is attributed to a degree of non-specific cross-tolerance between GTN and NP. 5. Co-treatment of a group of rats with N-acetyl-L-cysteine (L-NAC) prevented the development of nitrate tolerance, confirming the role of thiols in this phenomenon, whereas N-acetyl-D-cysteine (D-NAC) did not. 6. The stereospecificity in the effect of NAC in preventing this specific tolerance to GTN suggests that the interaction between GTN and NAC and/or cysteine involves an enzyme-dependent step. 7. NAC was unable to prevent the non-specific cross-tolerance to NP which followed infusion of GTN, suggesting that the mechanism does not directly involve NAC and/or cysteine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollah A., Moffat J. A., Armstrong P. W. N-acetylcysteine does not modify nitroglycerin-induced tolerance in canine vascular rings. J Cardiovasc Pharmacol. 1987 Apr;9(4):445–450. doi: 10.1097/00005344-198704000-00009. [DOI] [PubMed] [Google Scholar]
- Axelsson K. L., Andersson R. G. Tolerance towards nitroglycerin, induced in vivo, is correlated to a reduced cGMP response and an alteration in cGMP turnover. Eur J Pharmacol. 1983 Mar 18;88(1):71–79. doi: 10.1016/0014-2999(83)90393-x. [DOI] [PubMed] [Google Scholar]
- Axelsson K. L., Andersson R. G., Wikberg J. E. Vascular smooth muscle relaxation by nitro compounds: reduced relaxation and cGMP elevation in tolerant vessels and reversal of tolerance by dithiothreitol. Acta Pharmacol Toxicol (Copenh) 1982 May;50(5):350–357. doi: 10.1111/j.1600-0773.1982.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- Cotgreave I. A., Sandy M. S., Berggren M., Moldéus P. W., Smith M. T. N-acetylcysteine and glutathione-dependent protective effect of PZ51 (Ebselen) against diquat-induced cytotoxicity in isolated hepatocytes. Biochem Pharmacol. 1987 Sep 15;36(18):2899–2904. doi: 10.1016/0006-2952(87)90200-0. [DOI] [PubMed] [Google Scholar]
- Elkayam U., Kulick D., McIntosh N., Roth A., Hsueh W., Rahimtoola S. H. Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure. Circulation. 1987 Sep;76(3):577–584. doi: 10.1161/01.cir.76.3.577. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Lemke S. M. Dissociation of cysteine and glutathione levels from nitroglycerin-induced relaxation. Eur J Pharmacol. 1985 Apr 23;111(1):85–95. doi: 10.1016/0014-2999(85)90116-5. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Lemke S. M. Effects of sulfhydryl reagents on nitroglycerin-induced relaxation of bovine coronary artery. Can J Physiol Pharmacol. 1986 Nov;64(11):1395–1401. doi: 10.1139/y86-236. [DOI] [PubMed] [Google Scholar]
- Henry P. J., Horowitz J. D., Louis W. J. Nitroglycerin-induced tolerance affects multiple sites in the organic nitrate bioconversion cascade. J Pharmacol Exp Ther. 1989 Feb;248(2):762–768. [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Keith R. A., Burkman A. M., Sokoloski T. D., Fertel R. H. Vascular tolerance to nitroglycerin and cyclic GMP generation in rat aortic smooth muscle. J Pharmacol Exp Ther. 1982 Jun;221(3):525–531. [PubMed] [Google Scholar]
- May D. C., Popma J. J., Black W. H., Schaefer S., Lee H. R., Levine B. D., Hillis L. D. In vivo induction and reversal of nitroglycerin tolerance in human coronary arteries. N Engl J Med. 1987 Sep 24;317(13):805–809. doi: 10.1056/NEJM198709243171305. [DOI] [PubMed] [Google Scholar]
- Molina C. R., Andresen J. W., Rapoport R. M., Waldman S., Murad F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J Cardiovasc Pharmacol. 1987 Oct;10(4):371–378. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Busse R., Bassenge E. Desensitization of guanylate cyclase in nitrate tolerance does not impair endothelium-dependent responses. Eur J Pharmacol. 1988 Dec 13;158(3):191–198. doi: 10.1016/0014-2999(88)90066-0. [DOI] [PubMed] [Google Scholar]
- Needleman P., Jakschik B., Johnson E. M., Jr Sulfhydryl requirement for relaxation of vascular smooth muscle. J Pharmacol Exp Ther. 1973 Nov;187(2):324–331. [PubMed] [Google Scholar]
- Needleman P., Johnson E. M., Jr Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973 Mar;184(3):709–715. [PubMed] [Google Scholar]
- Needleman P. Tolerance to the vascular effects of glyceryl trinitrate. J Pharmacol Exp Ther. 1970 Jan;171(1):98–102. [PubMed] [Google Scholar]
- Packer M., Lee W. H., Kessler P. D., Gottlieb S. S., Medina N., Yushak M. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med. 1987 Sep 24;317(13):799–804. doi: 10.1056/NEJM198709243171304. [DOI] [PubMed] [Google Scholar]
- Romanin C., Kukovetz W. R. Tolerance to nitroglycerin is caused by reduced guanylate cyclase activation. J Mol Cell Cardiol. 1989 Jan;21(1):41–48. doi: 10.1016/0022-2828(89)91491-0. [DOI] [PubMed] [Google Scholar]
- Schröder H., Leitman D. C., Bennett B. M., Waldman S. A., Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther. 1988 May;245(2):413–418. [PubMed] [Google Scholar]
- Sheffner A. L., Medler E. M., Bailey K. R., Gallo D. G., Mueller A. J., Sarett H. P. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966 Oct;15(10):1523–1535. doi: 10.1016/0006-2952(66)90197-3. [DOI] [PubMed] [Google Scholar]
- Sjödin K., Nilsson E., Hallberg A., Tunek A. Metabolism of N-acetyl-L-cysteine. Some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem Pharmacol. 1989 Nov 15;38(22):3981–3985. doi: 10.1016/0006-2952(89)90677-1. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Ginsburg R., Murad F. Desensitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem Pharmacol. 1986 Oct 15;35(20):3525–3531. doi: 10.1016/0006-2952(86)90622-2. [DOI] [PubMed] [Google Scholar]
- Yeates R. A., Laufen H., Leitold M. The reaction between organic nitrates and sulfhydryl compounds. A possible model system for the activation of organic nitrates. Mol Pharmacol. 1985 Dec;28(6):555–559. [PubMed] [Google Scholar]


