Abstract
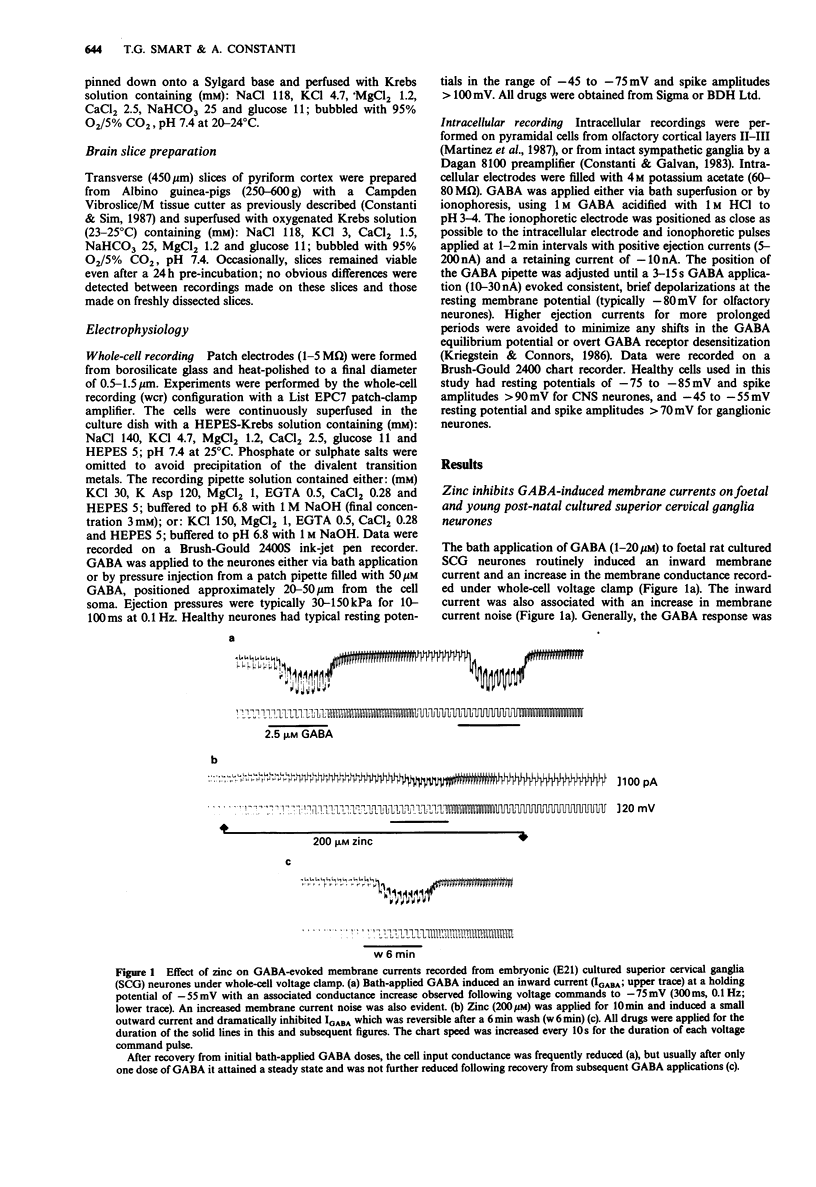
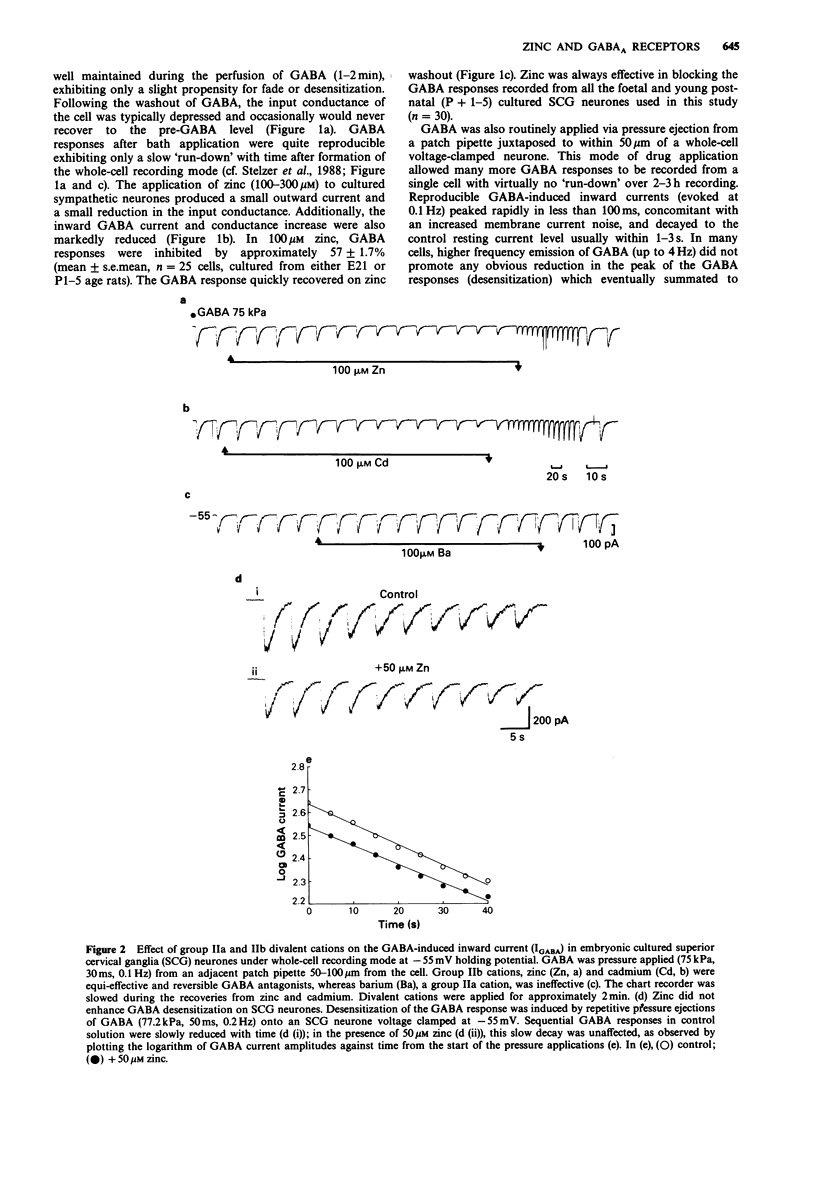
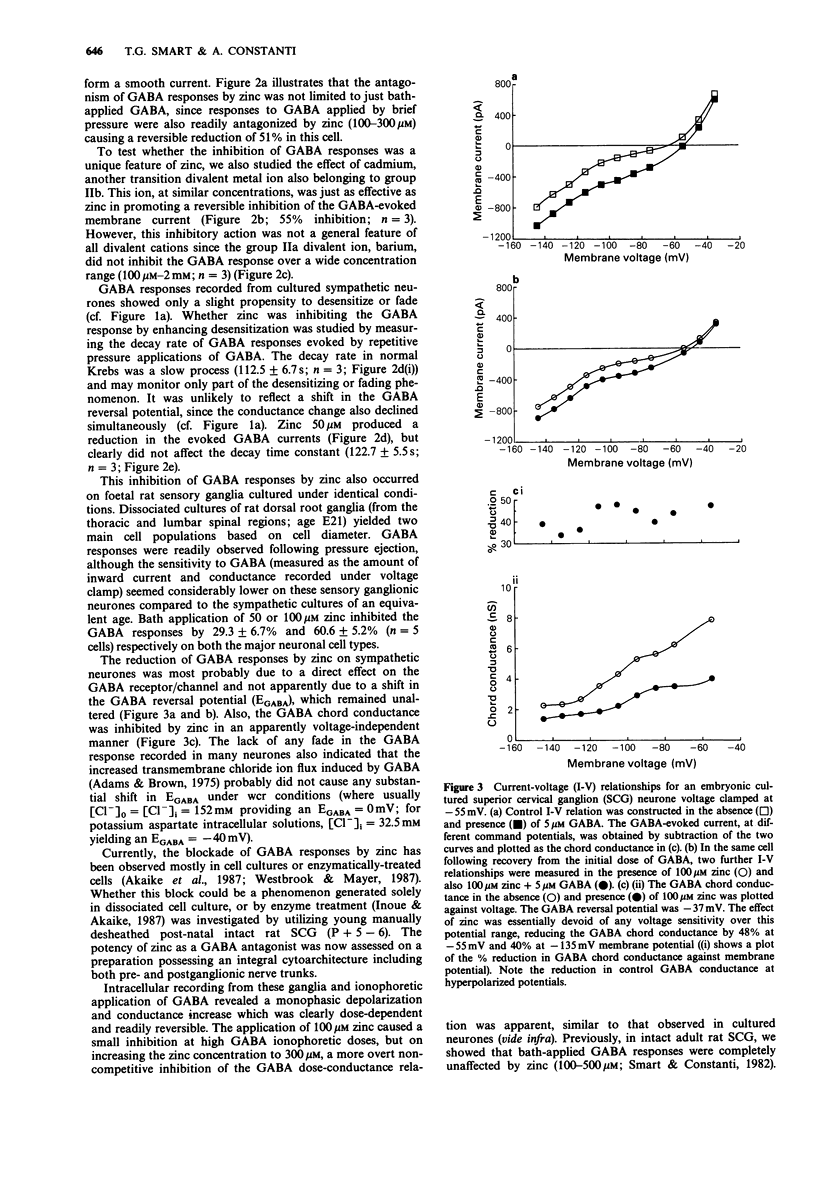
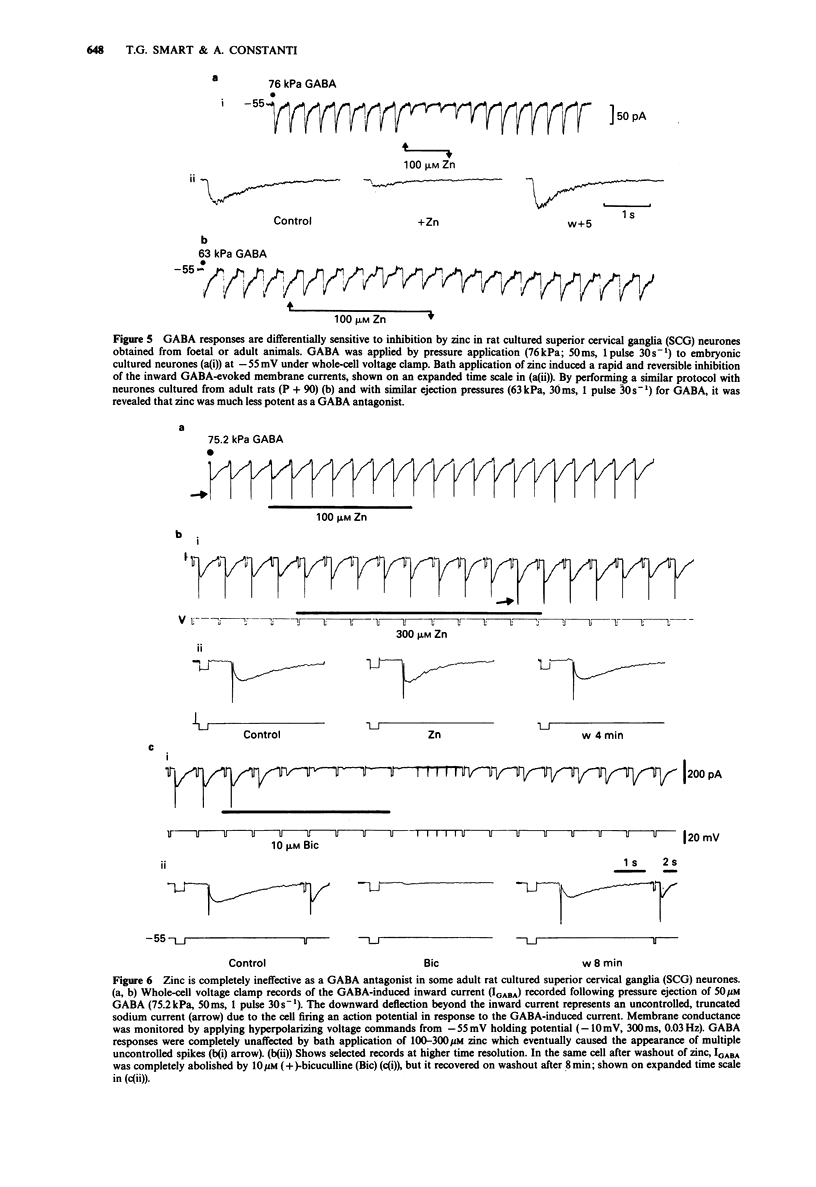
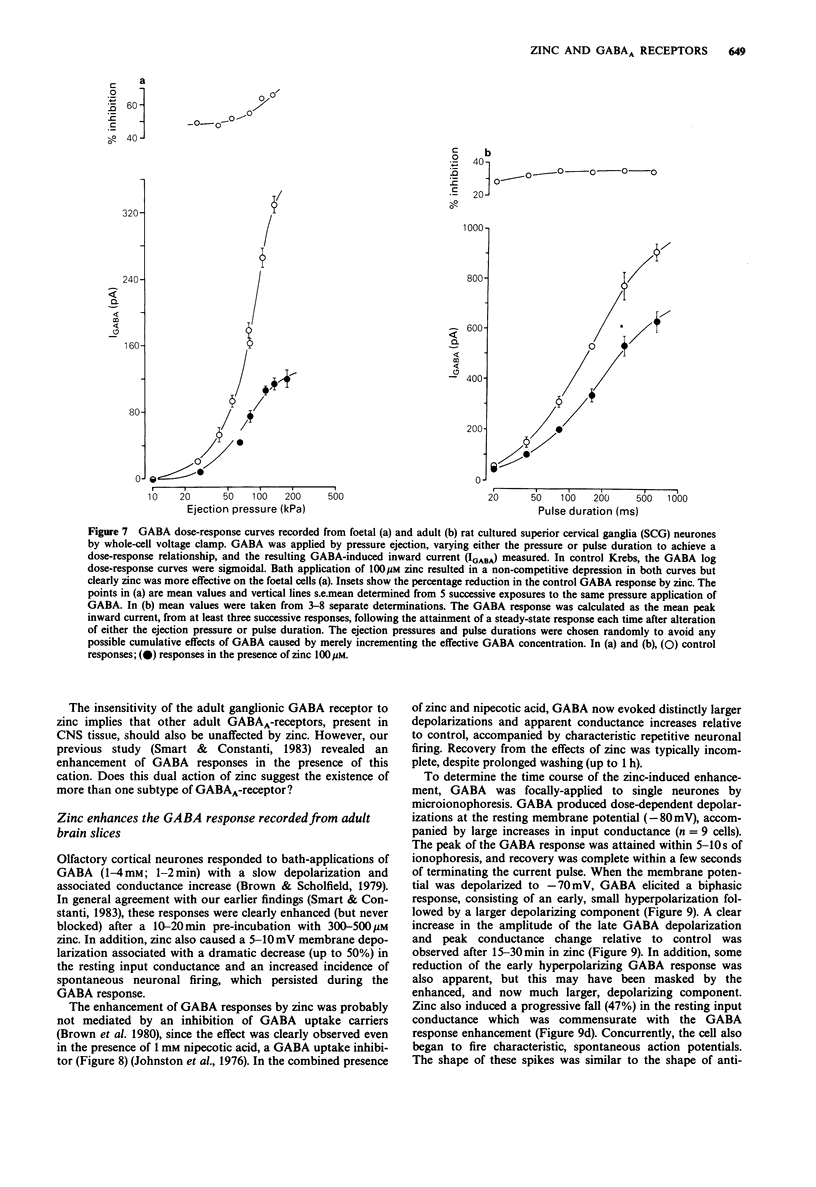
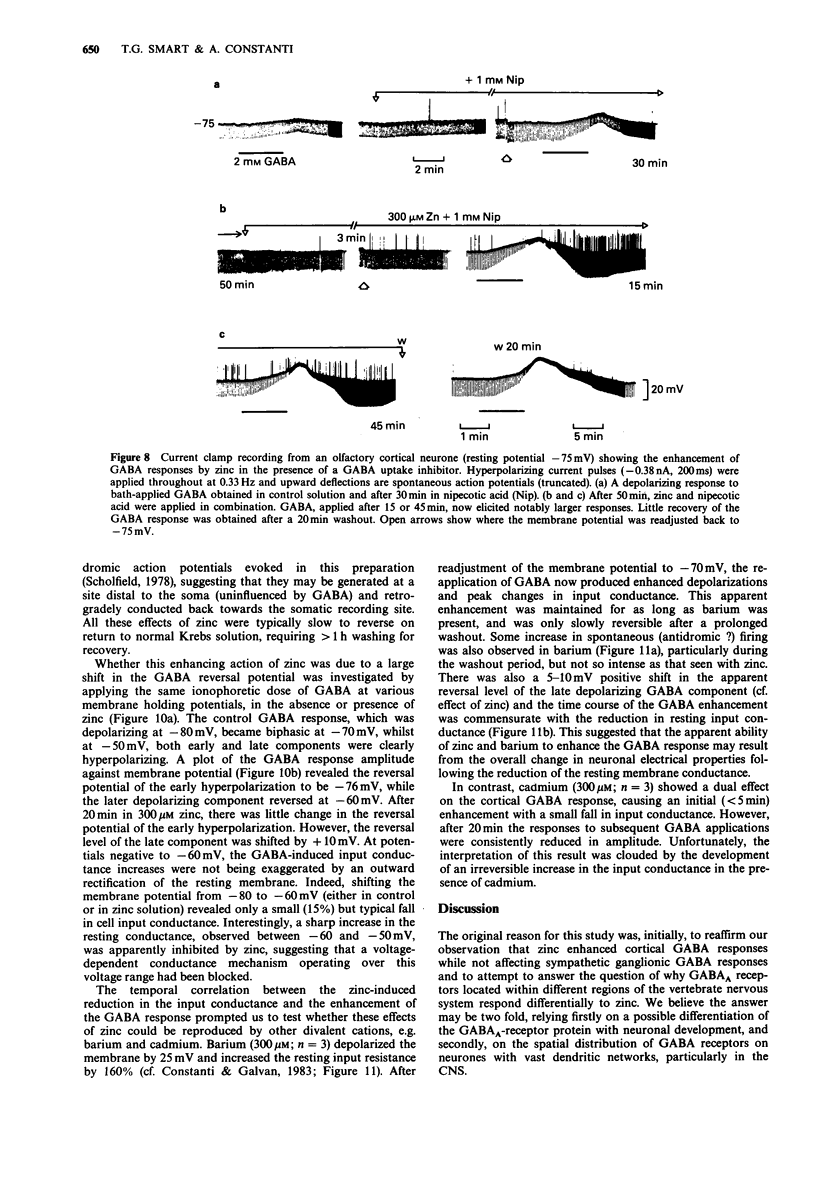
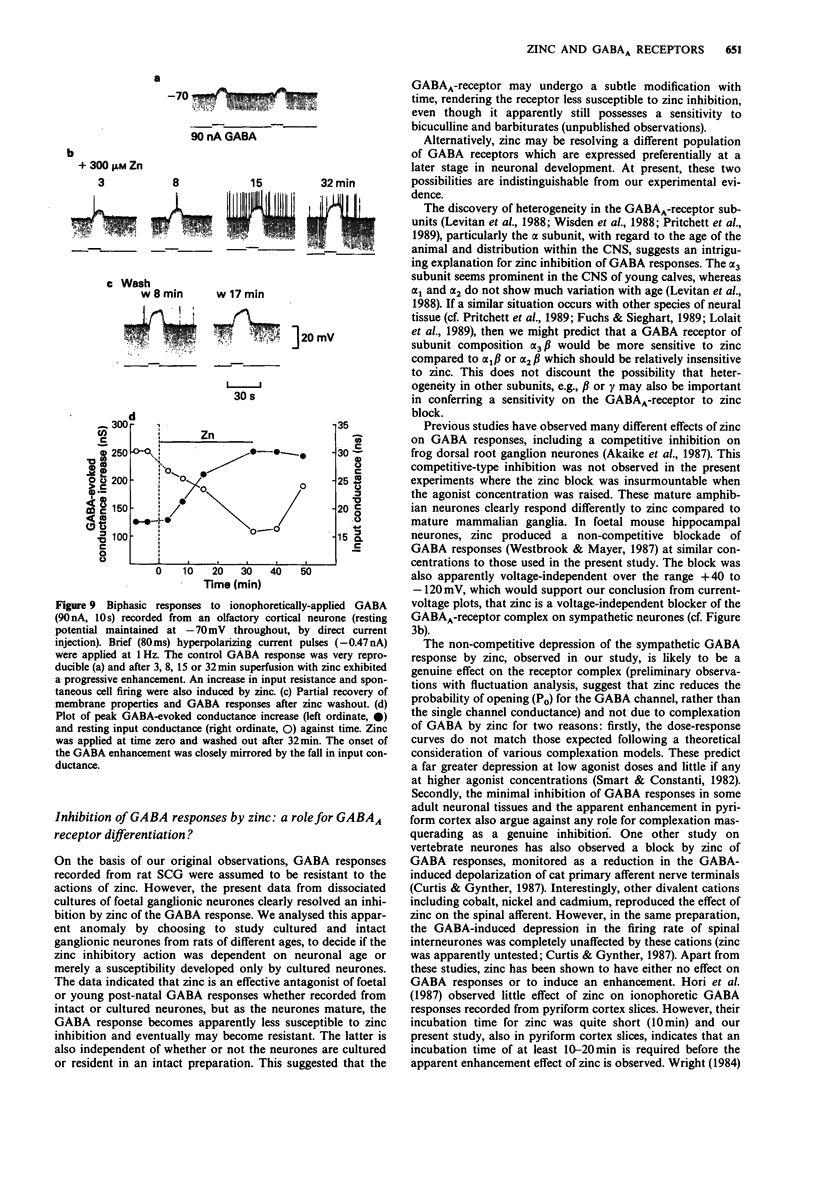
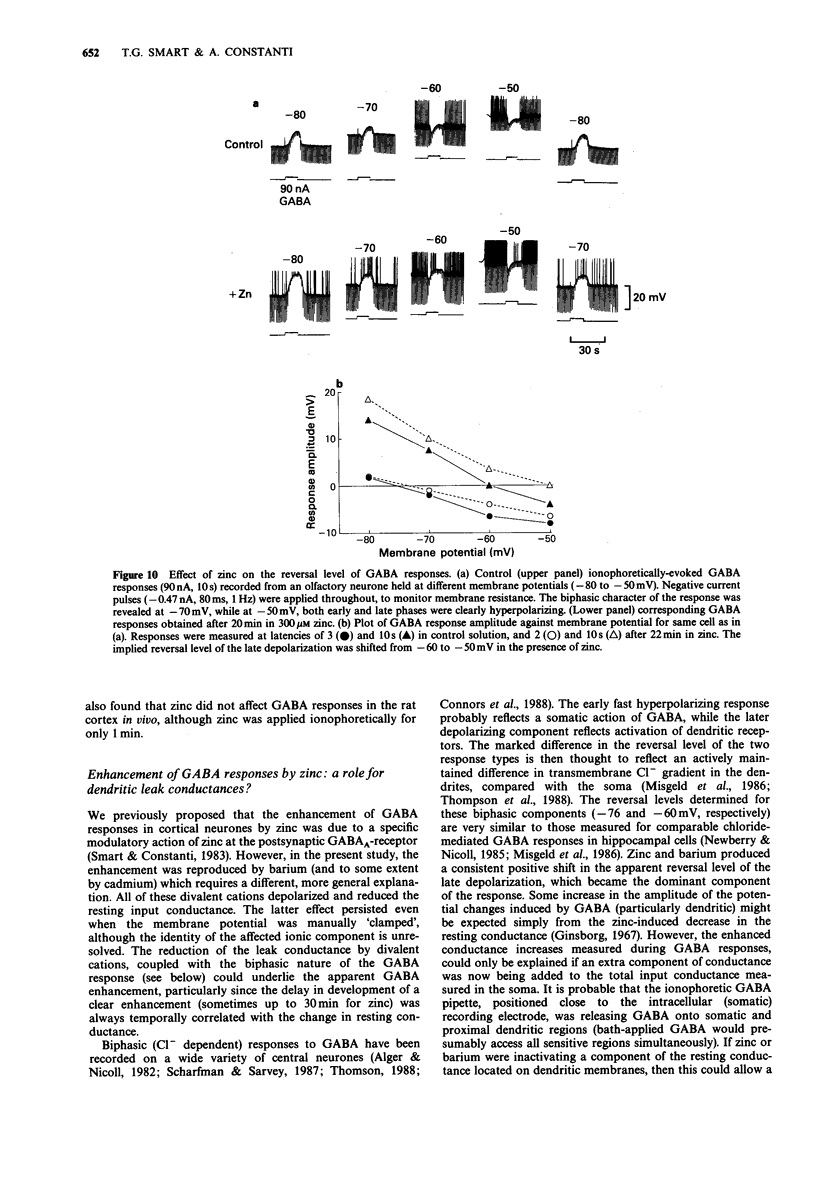
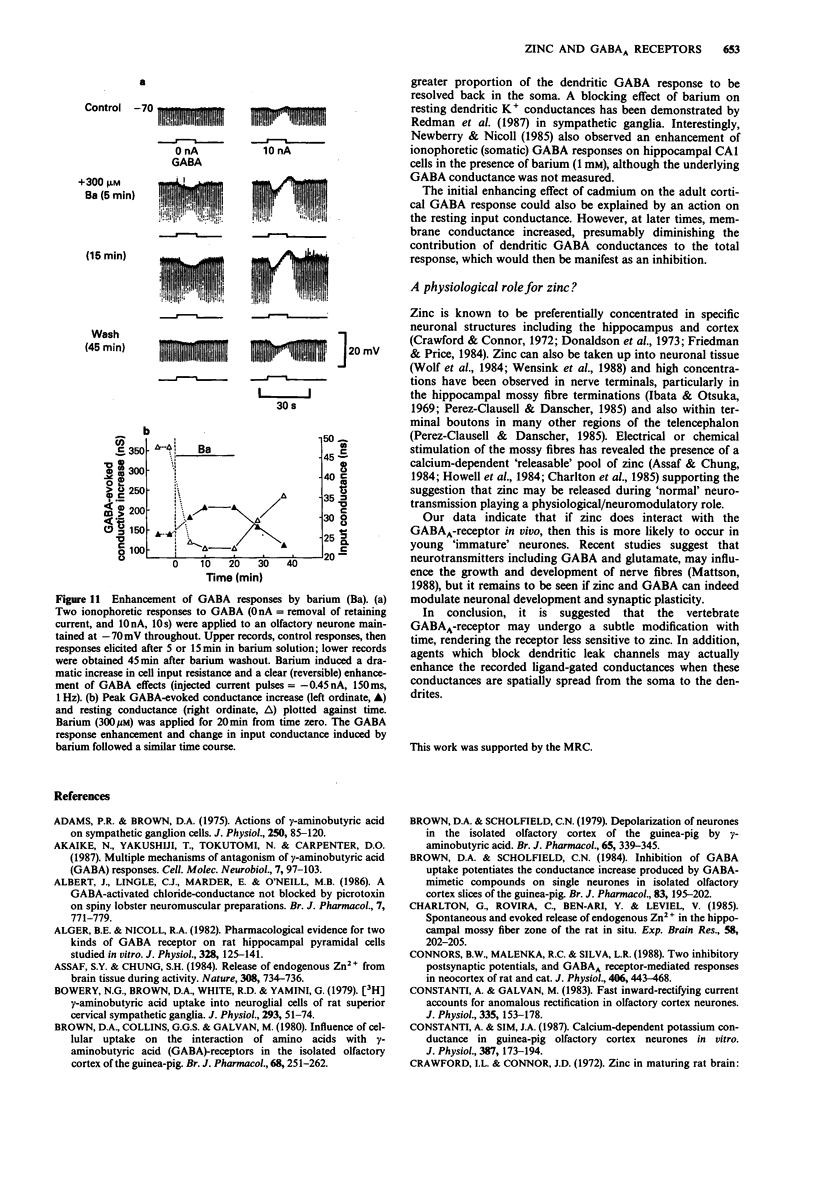
1. gamma-Aminobutyric acid (GABA) responses were recorded from rat superior cervical ganglia (SCG) in culture using the whole cell recording technique. 2. Zinc (50-300 microM) reversibly antagonized the GABA response in embryonic and young post-natal neurones, while neurones cultured from adult animals were far less sensitive and occasionally resistant to zinc blockade. Cadmium (100-300 microM) also antagonised the GABA response, while barium (100 microM-2 mM) was ineffective. 3. The differential blocking effect of zinc on cultured neurones of different ages also occurred in intact SCG tissue. 4. The GABA log dose-response curve constructed with foetal or adult cultured neurones was reduced in a non-competitive manner by zinc. This inhibition was minimally affected by the membrane potential. 5. The GABA response recorded intracellularly from guinea-pig pyriform cortical slices was enhanced by zinc (300-500 microM), which occurred concurrently with a decrease in the input conductance of the cell. The enhancement was unaffected by prior blockade of the GABA uptake carrier by 1 mM nipecotic acid. This phenomenon could be reproduced by barium (300 microM) and cadmium (300 microM). 6. We conclude that the vertebrate neuronal GABAA-receptor becomes less sensitive to zinc with neural (GABAA-receptor?) development, and the enhanced GABA response recorded in the CNS is a consequence of the reduction in the input conductance and not due to a direct effect on the receptor complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Yakushiji T., Tokutomi N., Carpenter D. O. Multiple mechanisms of antagonism of gamma-aminobutyric acid (GABA) responses. Cell Mol Neurobiol. 1987 Mar;7(1):97–103. doi: 10.1007/BF00734993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert J., Lingle C. J., Marder E., O'Neil M. B. A GABA-activated chloride-conductance not blocked by picrotoxin on spiny lobster neuromuscular preparations. Br J Pharmacol. 1986 Apr;87(4):771–779. doi: 10.1111/j.1476-5381.1986.tb14596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf S. Y., Chung S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984 Apr 19;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., White R. D., Yamini G. [3H]gamma-Aminobutyric acid uptake into neuroglial cells of rat superior cervical sympathetic ganglia. J Physiol. 1979 Aug;293:51–74. doi: 10.1113/jphysiol.1979.sp012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Depolarization of neurones in the isolated olfactory cortex of the guinea-pig by gamma-aminobutyric acid. Br J Pharmacol. 1979 Feb;65(2):339–345. doi: 10.1111/j.1476-5381.1979.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Inhibition of GABA uptake potentiates the conductance increase produced by GABA-mimetic compounds on single neurones in isolated olfactory cortex slices of the guinea-pig. Br J Pharmacol. 1984 Sep;83(1):195–202. doi: 10.1111/j.1476-5381.1984.tb10135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charton G., Rovira C., Ben-Ari Y., Leviel V. Spontaneous and evoked release of endogenous Zn2+ in the hippocampal mossy fiber zone of the rat in situ. Exp Brain Res. 1985;58(1):202–205. doi: 10.1007/BF00238969. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Sim J. A. Calcium-dependent potassium conductance in guinea-pig olfactory cortex neurones in vitro. J Physiol. 1987 Jun;387:173–194. doi: 10.1113/jphysiol.1987.sp016569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. L., Connor J. D. Zinc in maturing rat brain: hippocampal concentration and localization. J Neurochem. 1972 Jun;19(6):1451–1458. doi: 10.1111/j.1471-4159.1972.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Gynther B. D. Divalent cations reduce depolarization of primary afferent terminations by GABA. Brain Res. 1987 Sep 29;422(1):192–195. doi: 10.1016/0006-8993(87)90558-0. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Pierre T. S., Minnich J. L., Barbeau A. Determination of Na + , K + , Mg 2+ , Cu 2+ , Zn 2+ , and Mn 2+ in rat brain regions. Can J Biochem. 1973 Jan;51(1):87–92. doi: 10.1139/o73-010. [DOI] [PubMed] [Google Scholar]
- Duman R. S., Sweetnam P. M., Gallombardo P. A., Tallman J. F. Molecular biology of inhibitory amino acid receptors. Mol Neurobiol. 1987 Spring-Summer;1(1-2):155–189. doi: 10.1007/BF02935267. [DOI] [PubMed] [Google Scholar]
- Friedman B., Price J. L. Fiber systems in the olfactory bulb and cortex: a study in adult and developing rats, using the timm method with the light and electron microscope. J Comp Neurol. 1984 Feb 10;223(1):88–109. doi: 10.1002/cne.902230108. [DOI] [PubMed] [Google Scholar]
- Fuchs K., Sieghart W. Evidence for the existence of several different alpha- and beta-subunits of the GABA/benzodiazepine receptor complex from rat brain. Neurosci Lett. 1989 Feb 27;97(3):329–333. doi: 10.1016/0304-3940(89)90619-8. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Hori N., Galeno T., Carpenter D. O. Responses of pyriform cortex neurons to excitatory amino acids: voltage dependence, conductance changes, and effects of divalent cations. Cell Mol Neurobiol. 1987 Mar;7(1):73–90. doi: 10.1007/BF00734991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell G. A., Welch M. G., Frederickson C. J. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984 Apr 19;308(5961):736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Ibata Y., Otsuka N. Electron microscopic demonstration of zinc in the hippocampal formation using Timm's sulfide silver technique. J Histochem Cytochem. 1969 Mar;17(3):171–175. doi: 10.1177/17.3.171. [DOI] [PubMed] [Google Scholar]
- Inoue M., Akaike N. Selective effects of enzyme treatment used for dissociating single cells on their GABA-receptor activities. Neurosci Res. 1987 Oct;5(1):74–81. doi: 10.1016/0168-0102(87)90025-3. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Krogsgaard-Larsen P., Stephanson A. L., Twitchin B. Inhibition of the uptake of GABA and related amino acids in rat brain slices by the optical isomers of nipecotic acid. J Neurochem. 1976 May;26(5):1029–1032. doi: 10.1111/j.1471-4159.1976.tb06488.x. [DOI] [PubMed] [Google Scholar]
- Kriegstein A. R., Connors B. W. Cellular physiology of the turtle visual cortex: synaptic properties and intrinsic circuitry. J Neurosci. 1986 Jan;6(1):178–191. doi: 10.1523/JNEUROSCI.06-01-00178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., Kusano K., Muller J. M., Brownstein M. J., Mahan L. C. Cloning and expression of a novel rat GABAA receptor. FEBS Lett. 1989 Mar 27;246(1-2):145–148. doi: 10.1016/0014-5793(89)80271-6. [DOI] [PubMed] [Google Scholar]
- Martínez M. C., Blanco J., Bullón M. M., Agudo F. J. Structure of the piriform cortex of the adult rat. A Golgi study. J Hirnforsch. 1987;28(3):341–348. [PubMed] [Google Scholar]
- Mattson M. P. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988 Apr-Jun;472(2):179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Pérez-Clausell J., Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985 Jun 24;337(1):91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- Redman S. J., McLachlan E. M., Hirst G. D. Nonuniform passive membrane properties of rat lumbar sympathetic ganglion cells. J Neurophysiol. 1987 Mar;57(3):633–644. doi: 10.1152/jn.1987.57.3.633. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Sarvey J. M. Responses to GABA recorded from identified rat visual cortical neurons. Neuroscience. 1987 Nov;23(2):407–422. doi: 10.1016/0306-4522(87)90065-0. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Electrical properties of neurones in the olfactory cortex slice in vitro. J Physiol. 1978 Feb;275:535–546. doi: 10.1113/jphysiol.1978.sp012206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D. The GABAA receptor-gated ion channel: biochemical and pharmacological studies of structure and function. Biochem Pharmacol. 1988 Sep 15;37(18):3369–3375. doi: 10.1016/0006-2952(88)90684-3. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. A novel effect of zinc on the lobster muscle GABA receptor. Proc R Soc Lond B Biol Sci. 1982 Jun 22;215(1200):327–341. doi: 10.1098/rspb.1982.0045. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. Pre- and postsynaptic effects of zinc on in vitro prepyriform neurones. Neurosci Lett. 1983 Sep 30;40(2):205–211. doi: 10.1016/0304-3940(83)90303-8. [DOI] [PubMed] [Google Scholar]
- Smart T. G. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol. 1987 Aug;389:337–360. doi: 10.1113/jphysiol.1987.sp016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Deisz R. A., Prince D. A. Relative contributions of passive equilibrium and active transport to the distribution of chloride in mammalian cortical neurons. J Neurophysiol. 1988 Jul;60(1):105–124. doi: 10.1152/jn.1988.60.1.105. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Biphasic responses of thalamic neurons to GABA in isolated rat brain slices--II. Neuroscience. 1988 May;25(2):503–512. doi: 10.1016/0306-4522(88)90254-0. [DOI] [PubMed] [Google Scholar]
- Wensink J., Molenaar A. J., Woroniecka U. D., Van den Hamer C. J. Zinc uptake into synaptosomes. J Neurochem. 1988 Mar;50(3):782–789. doi: 10.1111/j.1471-4159.1988.tb02982.x. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Wisden W., Morris B. J., Darlison M. G., Hunt S. P., Barnard E. A. Distinct GABAA receptor alpha subunit mRNAs show differential patterns of expression in bovine brain. Neuron. 1988 Dec;1(10):937–947. doi: 10.1016/0896-6273(88)90151-1. [DOI] [PubMed] [Google Scholar]
- Wolf G., Schütte M., Römhild W. Uptake and subcellular distribution of 65zinc in brain structures during the postnatal development of the rat. Neurosci Lett. 1984 Oct 12;51(2):277–280. doi: 10.1016/0304-3940(84)90564-0. [DOI] [PubMed] [Google Scholar]
- Wright D. M. Zinc: effect and interaction with other cations in the cortex of the rat. Brain Res. 1984 Oct 8;311(2):343–347. doi: 10.1016/0006-8993(84)90097-0. [DOI] [PubMed] [Google Scholar]
- Yakushiji T., Tokutomi N., Akaike N., Carpenter D. O. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987 Sep;22(3):1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]


