Abstract
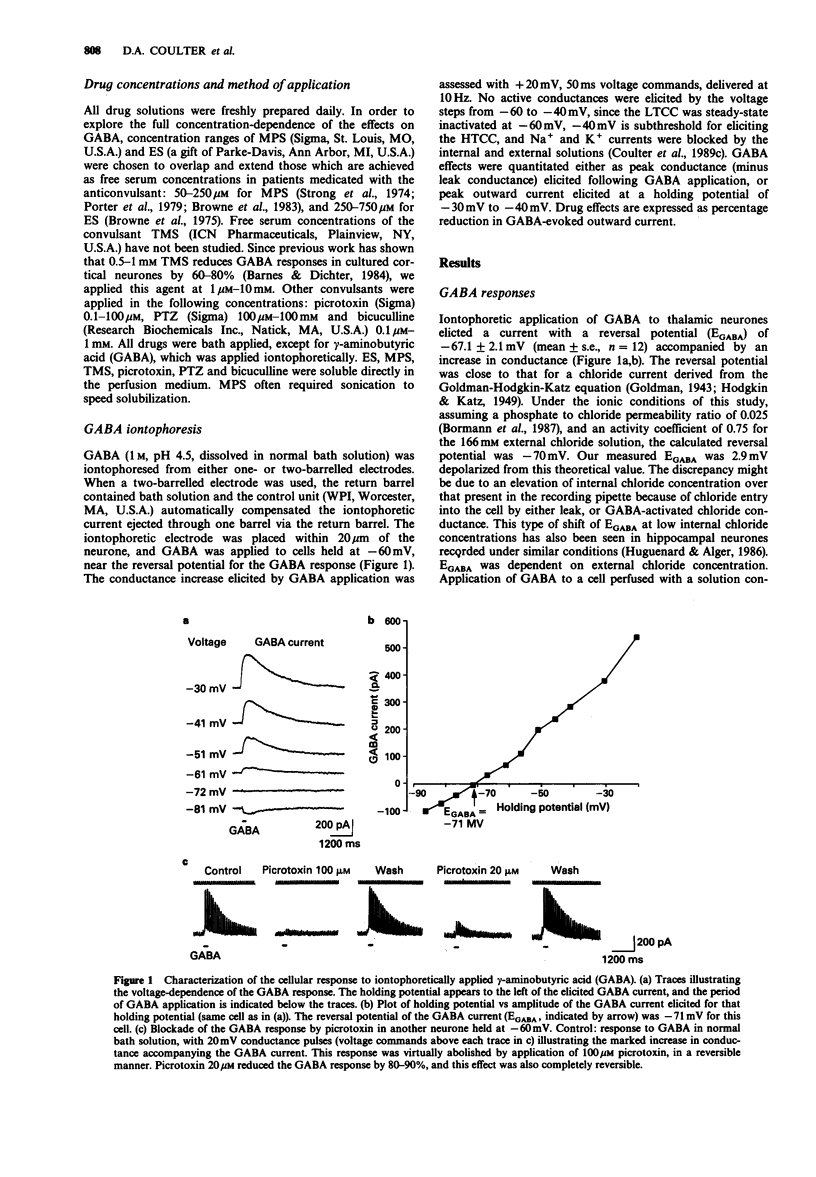
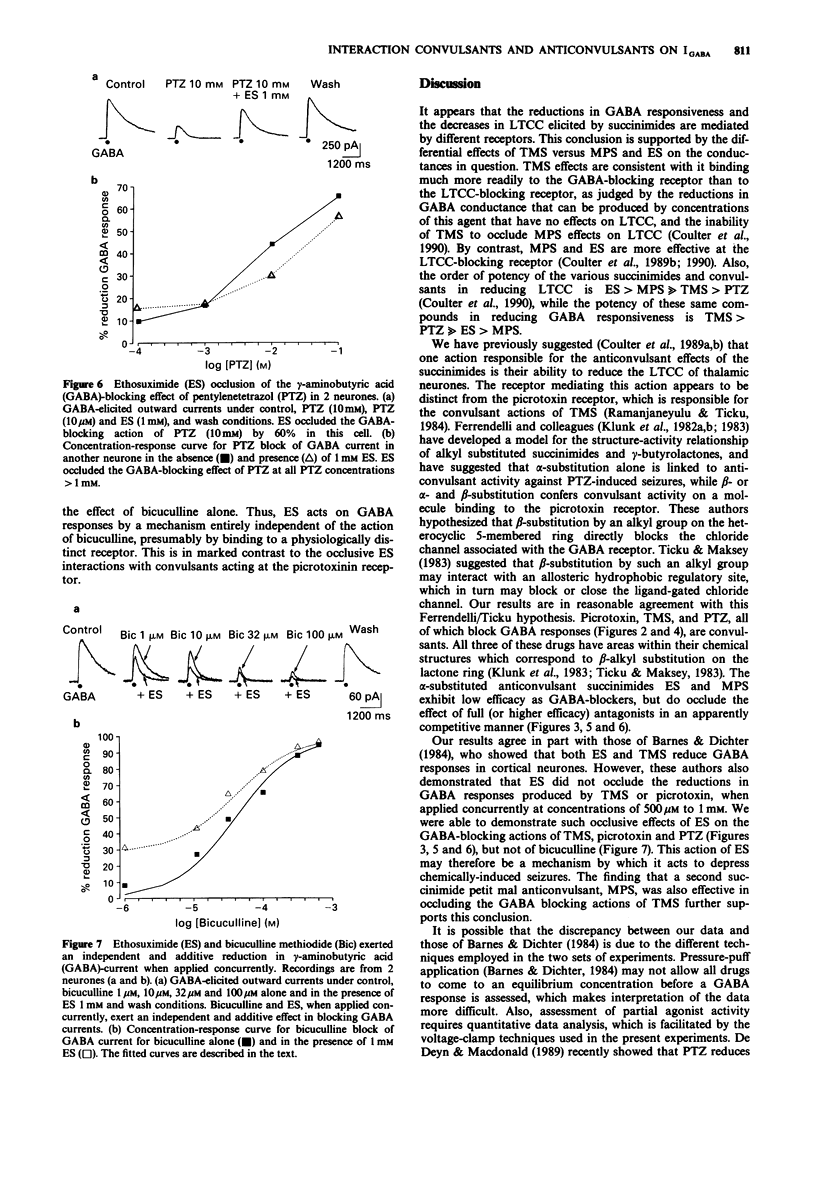
1. Currents evoked by applications of gamma-aminobutyric acid (GABA) to acutely dissociated thalamic neurones were analysed by voltage-clamp techniques, and the effects of the anticonvulsant succinimides ethosuximide (ES) and alpha-methyl-alpha-phenylsuccinimide (MPS) and the convulsants tetramethylsuccinimide (TMS), picrotoxin, pentylenetetrazol (PTZ), and bicuculline methiodide were assessed. 2. TMS (1 microM-10 microM) reduced responses to iontophoretically applied GABA, as did picrotoxin (0.1-100 microM), PTZ (1-100 mM) and bicuculline (1-100 microM). 3. ES, in high concentrations (1-10 mM), reduced GABA responses to a lesser extent, and also occluded the reductions in GABA-evoked currents produced by TMS, picrotoxin, and PTZ. ES did not occlude the effects of bicuculline on GABA responses. Therefore, we propose that ES acts as a partial agonist at the picrotoxin GABA-blocking receptor. 4. MPS had no effect on GABA responses (at a concentration of 1 mM), and, like ES, occluded the GABA-blocking actions of TMS, apparently acting as a full antagonist. 5. The anticonvulsant actions of ES and MPS against TMS and PTZ-induced seizures may thus involve two independent mechanisms: (1) the occlusion of TMS and PTZ GABA-blocking effects; and (2) the previously described specific effect of ES and MPS on low-threshold calcium current of thalamic neurones. The latter cellular mechanism may be more closely related to petit mal anticonvulsant activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Harrison N. L. Outward rectification of inhibitory postsynaptic currents in cultured rat hippocampal neurones. J Physiol. 1988 Sep;403:41–55. doi: 10.1113/jphysiol.1988.sp017237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. M., Dichter M. A. Effects of ethosuximide and tetramethylsuccinimide on cultured cortical neurons. Neurology. 1984 May;34(5):620–625. doi: 10.1212/wnl.34.5.620. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne T. R., Dreifuss F. E., Dyken P. R., Goode D. J., Penry J. K., Porter R. J., White B. G., White P. T. Ethosuximide in the treatment of absence (peptit mal) seizures. Neurology. 1975 Jun;25(6):515–524. doi: 10.1212/wnl.25.6.515. [DOI] [PubMed] [Google Scholar]
- Browne T. R., Feldman R. G., Buchanan R. A., Allen N. C., Fawcett-Vickers L., Szabo G. K., Mattson G. F., Norman S. E., Greenblatt D. J. Methsuximide for complex partial seizures: efficacy, toxicity, clinical pharmacology, and drug interactions. Neurology. 1983 Apr;33(4):414–418. doi: 10.1212/wnl.33.4.414. [DOI] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989 Jul;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989 Jun;25(6):582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Differential effects of petit mal anticonvulsants and convulsants on thalamic neurones: calcium current reduction. Br J Pharmacol. 1990 Aug;100(4):800–806. doi: 10.1111/j.1476-5381.1990.tb14095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Specific petit mal anticonvulsants reduce calcium currents in thalamic neurons. Neurosci Lett. 1989 Mar 13;98(1):74–78. doi: 10.1016/0304-3940(89)90376-5. [DOI] [PubMed] [Google Scholar]
- De Deyn P. P., Macdonald R. L. Effects of antiepileptic drugs on GABA responses and on reduction of GABA responses by PTZ and DMCM on mouse neurons in cell culture. Epilepsia. 1989 Jan-Feb;30(1):17–25. doi: 10.1111/j.1528-1157.1989.tb05275.x. [DOI] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Alger B. E. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986 Jul;56(1):1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Anticonvulsant properties of alpha, gamma, and alpha, gamma-substituted gamma-butyrolactones. Mol Pharmacol. 1982 Sep;22(2):438–443. [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Comparison of epileptogenic properties of unsubstituted and beta-alkyl-substituted gamma-butyrolactones. Mol Pharmacol. 1982 Sep;22(2):431–437. [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Structure-activity relationships of alkyl-substituted gamma-butyrolactones and succinimides. Mol Pharmacol. 1982 Sep;22(2):444–450. [PubMed] [Google Scholar]
- Klunk W. E., Kalman B. L., Ferrendelli J. A., Covey D. F. Computer-assisted modeling of the picrotoxinin and gamma-butyrolactone receptor site. Mol Pharmacol. 1983 Mar;23(2):511–518. [PubMed] [Google Scholar]
- Löscher W., Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988 May-Jun;2(3):145–181. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Barker J. L. Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurones. Nature. 1977 Jun 23;267(5613):720–721. doi: 10.1038/267720a0. [DOI] [PubMed] [Google Scholar]
- Maksay G., Ticku M. K. Dissociation of [35S]t-butylbicyclophosphorothionate binding differentiates convulsant and depressant drugs that modulate GABAergic transmission. J Neurochem. 1985 Feb;44(2):480–486. doi: 10.1111/j.1471-4159.1985.tb05439.x. [DOI] [PubMed] [Google Scholar]
- Mody I., Salter M. W., MacDonald J. F. Requirement of NMDA receptor/channels for intracellular high-energy phosphates and the extent of intraneuronal calcium buffering in cultured mouse hippocampal neurons. Neurosci Lett. 1988 Oct 31;93(1):73–78. doi: 10.1016/0304-3940(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Padjen A. Pentylenetetrazol: an antagonist of GABA at primary afferents of the isolated frog spinal cord. Neuropharmacology. 1976 Jan;15(1):69–71. doi: 10.1016/0028-3908(76)90099-x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Ramanjaneyulu R., Ticku M. K. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complex. Eur J Pharmacol. 1984 Mar 2;98(3-4):337–345. doi: 10.1016/0014-2999(84)90282-6. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: properties of GABA-activated Cl- ion conductance. J Neurophysiol. 1984 Mar;51(3):500–515. doi: 10.1152/jn.1984.51.3.500. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Classification of some GABA antagonists with regard to site of action and potency in slices of rat cuneate nucleus. Eur J Pharmacol. 1982 Jun 4;80(4):347–358. doi: 10.1016/0014-2999(82)90080-2. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Evidence that bicuculline and picrotoxin act at separate sites to antagonize gamma-aminobutyric acid in rat cuneate nucleus. Neuropharmacology. 1980 Jan;19(1):39–45. doi: 10.1016/0028-3908(80)90164-1. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Casida J. E., Richardson M., Saederup E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983 Mar;23(2):326–336. [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Strong J. M., Abe T., Gibbs E. L., Atkinson A. J., Jr Plasma levels of methsuximide and N-desmethylmethsuximide during methsuximide therapy. Neurology. 1974 Mar;24(3):250–255. doi: 10.1212/wnl.24.3.250. [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Maksay G. Convulsant/depressant site of action at the allosteric benzodiazepine-GABA receptor-ionophore complex. Life Sci. 1983 Dec 12;33(24):2363–2375. doi: 10.1016/0024-3205(83)90630-6. [DOI] [PubMed] [Google Scholar]
- Trifiletti R. R., Snowman A. M., Snyder S. H. Barbiturate recognition site on the GABA/benzodiazepine receptor complex is distinct from the picrotoxinin/TBPS recognition site. Eur J Pharmacol. 1984 Nov 13;106(2):441–447. doi: 10.1016/0014-2999(84)90737-4. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Snowman A. M., Leeb-Lundberg L. M., Olsen R. W. Barbiturates allosterically inhibit GABA antagonist and benzodiazepine inverse agonist binding. Eur J Pharmacol. 1984 Jul 13;102(2):205–212. doi: 10.1016/0014-2999(84)90252-8. [DOI] [PubMed] [Google Scholar]


