Abstract
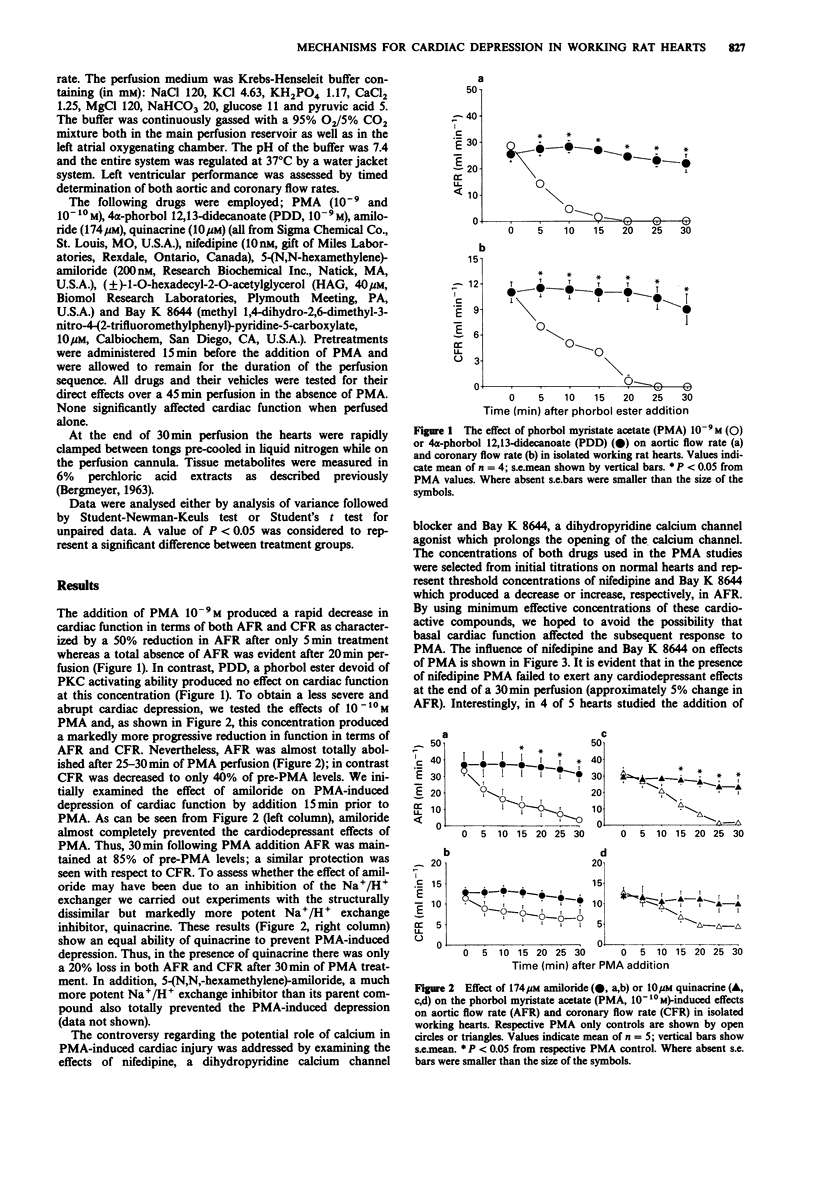
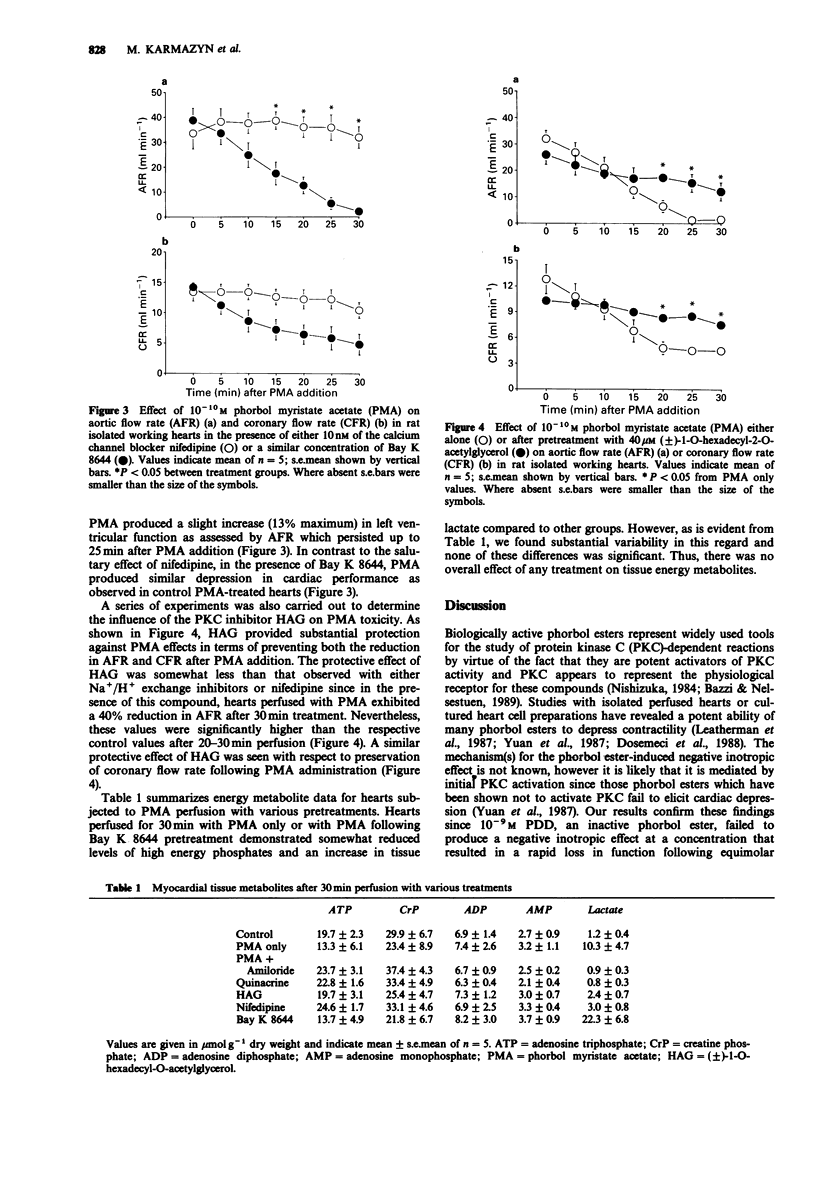
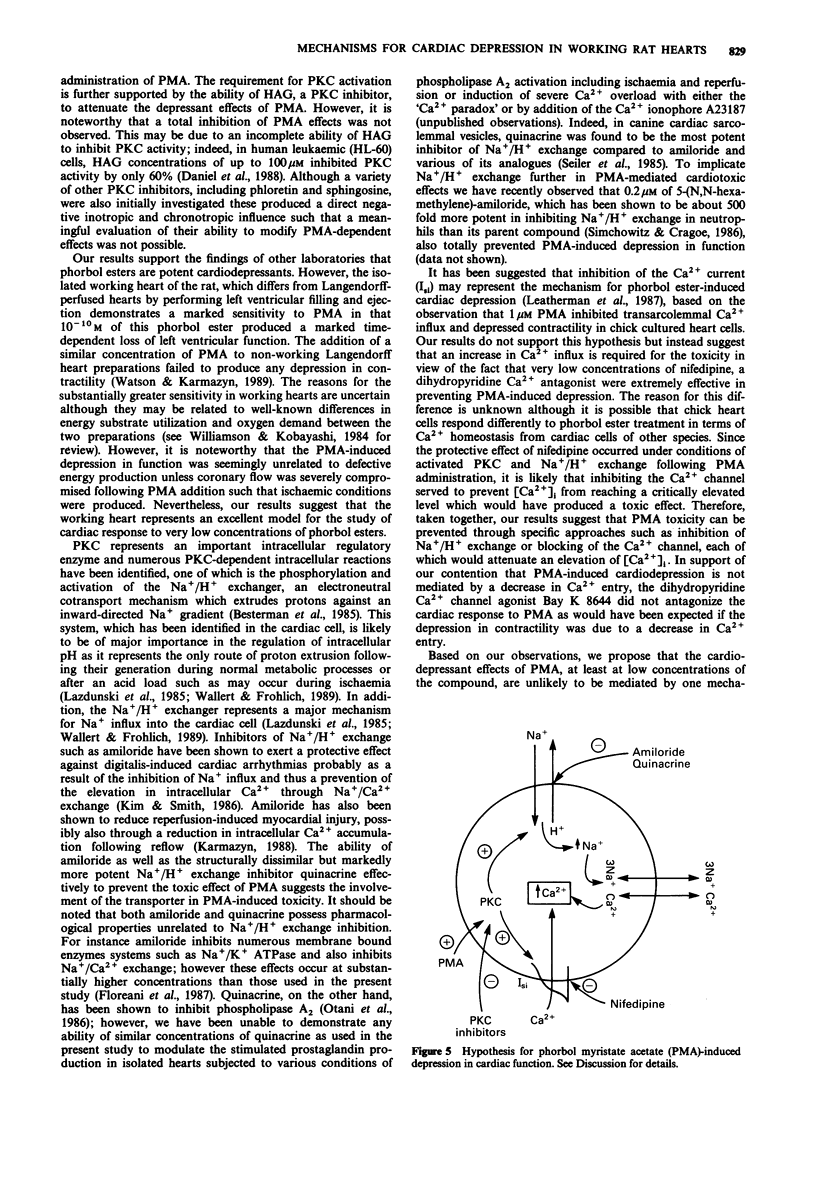
1. The effects of the phorbol ester, phorbol myristate acetate (PMA) were examined on function and energy metabolism in the isolated working heart of the rat. 2. At a concentration of 10(-9) M PMA produced a rapid loss in cardiac function in terms of aortic flow rate (AFR) and coronary flow rates (CFR) whereas a similar concentration of 4 alpha-phorbol 12,13-didecanoate was ineffective. At a concentration of 10(-10) M, the PMA-induced depression was more gradual but nevertheless very pronounced with an almost total loss in AFR after 30 min perfusion. The reduction in CFR was more moderate than that observed with respect to AFR. 3. The protein kinase C (PKC) inhibitor (+/-)-1-O-hexadecyl-2-O-acylglycerol significantly attenuated the loss in AFR and CFR following addition of PMA. 4. Two inhibitors of Na+/H+ exchange, amiloride and quinacrine, totally prevented the reduction in AFR. Although the PMA-induced depression in CFR was also attenuated by both amiloride and quinacrine, these effects were not significant, probably reflecting the less pronounced effect of PMA on this parameter. 5. Nifedipine, a dihydropyridine calcium channel blocker reduced PMA toxicity to a similar degree as Na+/N+ exchange inhibition whereas the calcium channel agonist Bay K 8644 was without effect. 6. Tissue content of energy metabolites including high energy phosphates, total adenine nucleotides or lactate were not significantly affected by PMA perfusion. 7. We conclude that PKC activation is necessary for phorbol ester-induced cardiac dysfunction.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Nelsestuen G. L. Properties of the protein kinase C-phorbol ester interaction. Biochemistry. 1989 Apr 18;28(8):3577–3585. doi: 10.1021/bi00434a064. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., May W. S., Jr, LeVine H., 3rd, Cragoe E. J., Jr, Cuatrecasas P. Amiloride inhibits phorbol ester-stimulated Na+/H+ exchange and protein kinase C. An amiloride analog selectively inhibits Na+/H+ exchange. J Biol Chem. 1985 Jan 25;260(2):1155–1159. [PubMed] [Google Scholar]
- Daniel L. W., Small G. W., Schmitt J. D., Marasco C. J., Ishaq K., Piantadosi C. Alkyl-linked diglycerides inhibit protein kinase C activation by diacylglycerols. Biochem Biophys Res Commun. 1988 Feb 29;151(1):291–297. doi: 10.1016/0006-291x(88)90592-x. [DOI] [PubMed] [Google Scholar]
- Döemeci A., Dhallan R. S., Cohen N. M., Lederer W. J., Rogers T. B. Phorbol ester increases calcium current and simulates the effects of angiotensin II on cultured neonatal rat heart myocytes. Circ Res. 1988 Feb;62(2):347–357. doi: 10.1161/01.res.62.2.347. [DOI] [PubMed] [Google Scholar]
- Floreani M., Tessari M., Debetto P., Luciani S., Carpenedo F. Effects of N-chlorobenzyl analogues of amiloride on myocardial contractility, Na-Ca-exchange carrier and other cardiac enzymatic activities. Naunyn Schmiedebergs Arch Pharmacol. 1987 Dec;336(6):661–669. doi: 10.1007/BF00165758. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Chang F. C., O'Callahan C. M., Ptasienski J. L-type calcium channels in cardiac and skeletal muscle. Purification and phosphorylation. Ann N Y Acad Sci. 1989;560:27–38. doi: 10.1111/j.1749-6632.1989.tb24076.x. [DOI] [PubMed] [Google Scholar]
- Karmazyn M. Amiloride enhances postischemic ventricular recovery: possible role of Na+-H+ exchange. Am J Physiol. 1988 Sep;255(3 Pt 2):H608–H615. doi: 10.1152/ajpheart.1988.255.3.H608. [DOI] [PubMed] [Google Scholar]
- Karmazyn M., Neely J. R. Inhibition of post-ischemic ventricular recovery by low concentrations of prostacyclin in isolated working rat hearts: dependency on concentration, ischemia duration, calcium and relationship to myocardial energy metabolism. J Mol Cell Cardiol. 1989 Mar;21(3):335–346. doi: 10.1016/0022-2828(89)90749-9. [DOI] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Effects of amiloride and ouabain on contractile state, Ca and Na fluxes, and Na content in cultured chick heart cells. Mol Pharmacol. 1986 Apr;29(4):363–371. [PubMed] [Google Scholar]
- Lazdunski M., Frelin C., Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985 Nov;17(11):1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- Leatherman G. F., Kim D., Smith T. W. Effect of phorbol esters on contractile state and calcium flux in cultured chick heart cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H205–H209. doi: 10.1152/ajpheart.1987.253.1.H205. [DOI] [PubMed] [Google Scholar]
- Limas C. J., Limas C. Phorbol ester- and diacylglycerol-mediated desensitization of cardiac beta-adrenergic receptors. Circ Res. 1985 Sep;57(3):443–449. doi: 10.1161/01.res.57.3.443. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J. Techniques for perfusing isolated rat hearts. Methods Enzymol. 1975;39:43–60. doi: 10.1016/s0076-6879(75)39008-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Otani H., Engelman R. M., Breyer R. H., Rousou J. A., Lemeshow S., Das D. K. Mepacrine, a phospholipase inhibitor. A potential tool for modifying myocardial reperfusion injury. J Thorac Cardiovasc Surg. 1986 Aug;92(2):247–254. [PubMed] [Google Scholar]
- Satoh H., Hashimoto K. On electrophysiological responses to phorbol esters which stimulate protein kinase C in rabbit sino-atrial node cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Mar;337(3):308–315. doi: 10.1007/BF00168844. [DOI] [PubMed] [Google Scholar]
- Seiler S. M., Cragoe E. J., Jr, Jones L. R. Demonstration of a Na+/H+ exchange activity in purified canine cardiac sarcolemmal vesicles. J Biol Chem. 1985 Apr 25;260(8):4869–4876. [PubMed] [Google Scholar]
- Simchowitz L., Cragoe E. J., Jr Inhibition of chemotactic factor-activated Na+/H+ exchange in human neutrophils by analogues of amiloride: structure-activity relationships in the amiloride series. Mol Pharmacol. 1986 Aug;30(2):112–120. [PubMed] [Google Scholar]
- Wallert M. A., Fröhlich O. Na+-H+ exchange in isolated myocytes from adult rat heart. Am J Physiol. 1989 Aug;257(2 Pt 1):C207–C213. doi: 10.1152/ajpcell.1989.257.2.C207. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kobayashi K. Use of the perfused rat heart to study cardiac metabolism: retrospective and prospective views. Basic Res Cardiol. 1984 May-Jun;79(3):283–291. doi: 10.1007/BF01908028. [DOI] [PubMed] [Google Scholar]
- Yuan S. H., Sunahara F. A., Sen A. K. Tumor-promoting phorbol esters inhibit cardiac functions and induce redistribution of protein kinase C in perfused beating rat heart. Circ Res. 1987 Sep;61(3):372–378. doi: 10.1161/01.res.61.3.372. [DOI] [PubMed] [Google Scholar]


