Abstract
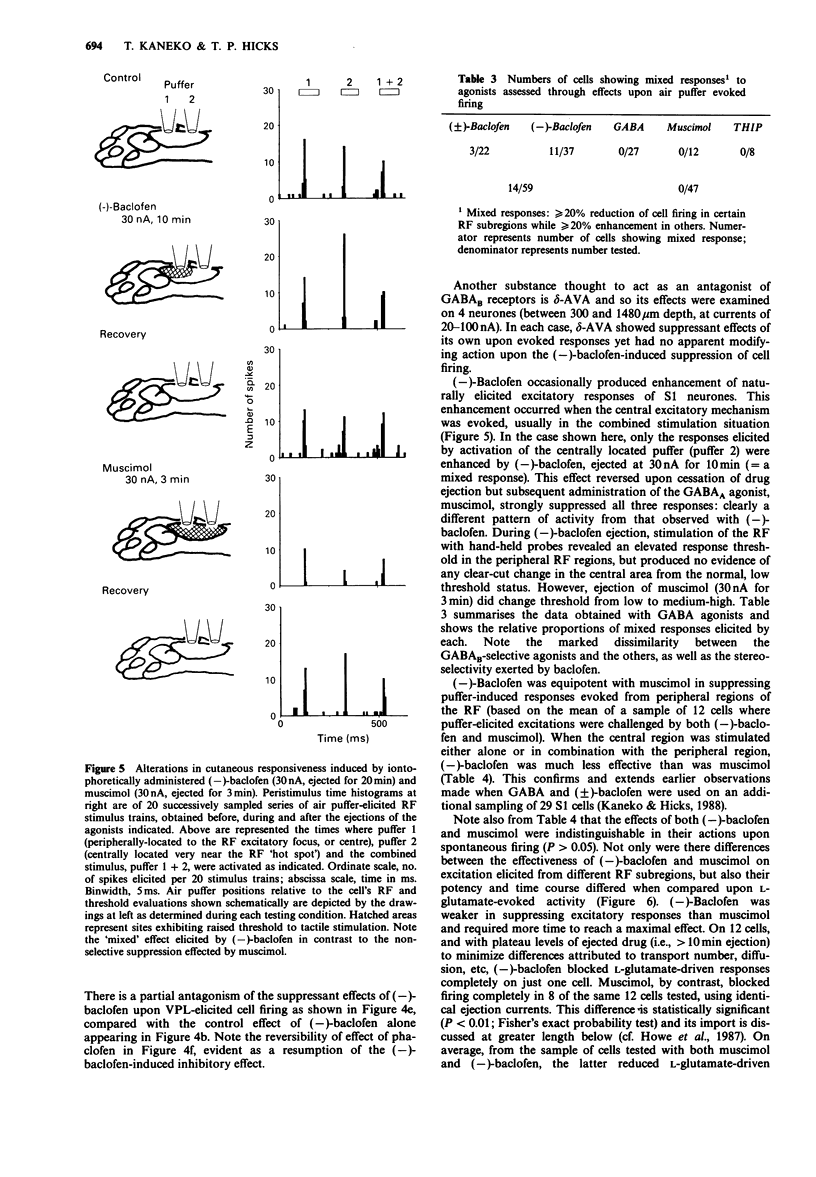
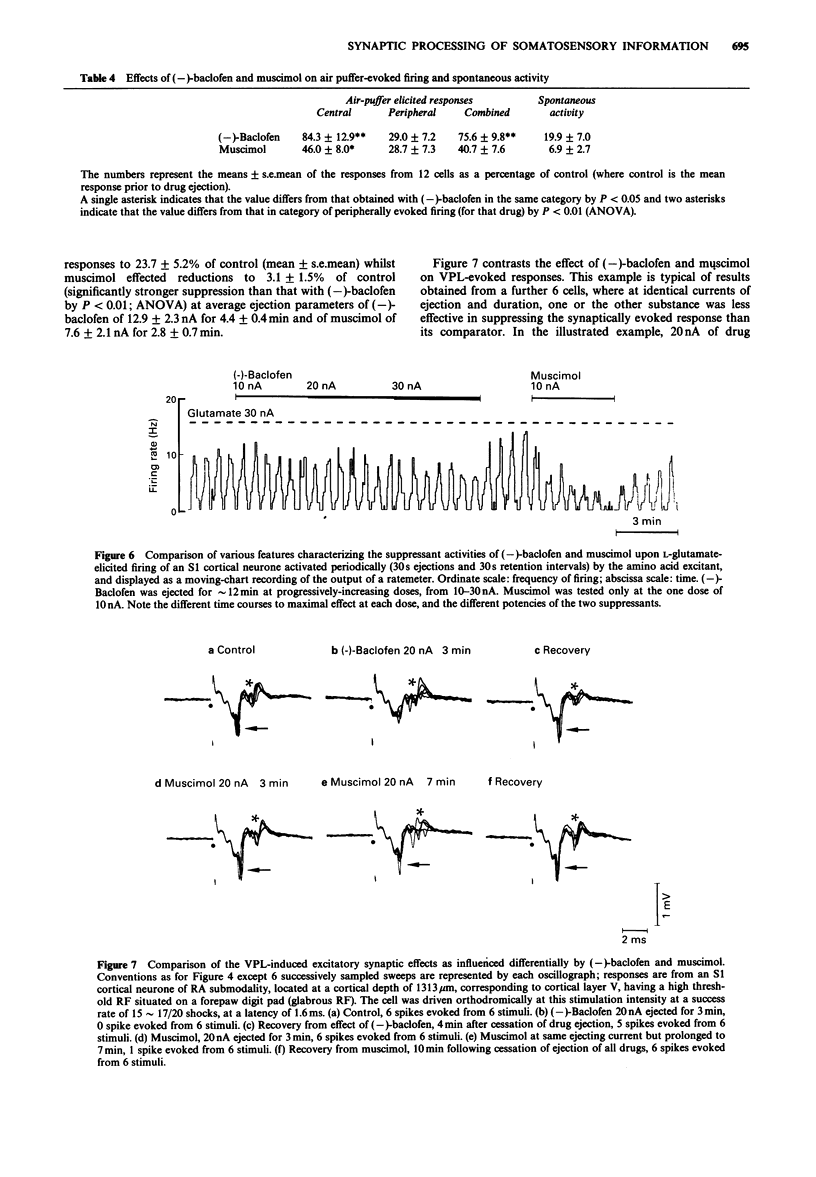
1. The possible role of GABA(B) receptor mechanisms in information processing in primary somatosensory (S1) cortex was assessed by use of extracellular recording combined with microiontophoretic methods from 161 neurones in anaesthetized, paralysed cats. 2. Baclofen-induced suppressions of cell responses were reversible and stereoselective, the (+)-isomer being inactive and the (-)-isomer having two to three times the apparent potency of gamma-aminobutyric acid (GABA). The responses measured were threshold to natural stimulation of receptive fields (RFs), responsiveness to thalamic electrical stimulation, change in RF size and magnitude of firing elicited by iontophoretic glutamate. 3. The action of GABA always was mimicked by muscimol or 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) but not always by (-)-baclofen; in certain cases (-)-baclofen enhanced neuronal responses while the opposite occurred with GABA or with the other GABA(A) agonists. The elevation of response thresholds by (-)-baclofen was relatively stronger in peripheral than in central subregions of cutaneous RFs, by contrast with the action of muscimol which was relatively non-selective as to the area in which it was effective. 4. Glutamate-induced and thalamically-evoked cortical responses as well as spontaneous activity were differentially sensitive to the suppressant effects of muscimol and (-)-baclofen. 5. Bicuculline methiodide reversibly blocked THIP- and muscimol-induced suppressions of tactile- (air puffer)-induced S1 responses but spared those produced by (-)-baclofen. Phaclofen and delta-amino-n-valeric acid were essentially inactive as blockers of (-)-baclofen-induced effects and in fact often acted as (-)-baclofen-like agonists, phaclofen being considerably weaker than delta-amino-n-valeric acid in this respect. 6. The range of suppressant effects produced by GABA as well as by muscimol and THIP, considered in conjunction with the actions of bicuculline methiodide, suggest that the effects observed by ejected GABA are likely to be due principally to GABA(A) processes, those mediated by GABA(B) receptors largely being masked. However, GABA(B) mechanisms are extant and do appear to be active, probably presynaptically and probably at sites distal to the soma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloway K. D., Burton H. Bicuculline-induced alterations in neuronal responses to controlled tactile stimuli in the second somatosensory cortex of the cat: a microiontophoretic study. Somatosens Res. 1986;3(3):197–211. doi: 10.3109/07367228609144584. [DOI] [PubMed] [Google Scholar]
- Alloway K. D., Rosenthal P., Burton H. Quantitative measurements of receptive field changes during antagonism of GABAergic transmission in primary somatosensory cortex of cats. Exp Brain Res. 1989;78(3):514–532. doi: 10.1007/BF00230239. [DOI] [PubMed] [Google Scholar]
- Alloway K. D., Sinclair R. J., Burton H. Responses of neurons in somatosensory cortical area II of cats to high-frequency vibratory stimuli during iontophoresis of a GABA antagonist and glutamate. Somatosens Mot Res. 1988;6(2):109–140. doi: 10.3109/08990228809144670. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. High affinity uptake of transmitters: studies on the uptake of L-aspartate, GABA, L-glutamate and glycine in cat spinal cord. J Neurochem. 1973 Feb;20(2):529–539. doi: 10.1111/j.1471-4159.1973.tb12152.x. [DOI] [PubMed] [Google Scholar]
- Batuev A. S., Alexandrov A. A., Scheynikov N. A. Picrotoxin action on the receptive fields of the cat sensorimotor cortex neurons. J Neurosci Res. 1982;7(1):49–55. doi: 10.1002/jnr.490070106. [DOI] [PubMed] [Google Scholar]
- Baumfalk U., Albus K. Phaclofen antagonizes baclofen-induced suppression of visually evoked responses in the cat's striate cortex. Brain Res. 1988 Nov 1;463(2):398–402. doi: 10.1016/0006-8993(88)90418-0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Malenka R. C., Silva L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988 Dec;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Bornstein J. C., Peet M. J. Selective effects of (-)-baclofen on spinal synaptic transmission in the cat. Exp Brain Res. 1981;42(2):158–170. doi: 10.1007/BF00236902. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Prince D. A. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989 May;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dykes R. W., Landry P., Metherate R., Hicks T. P. Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J Neurophysiol. 1984 Dec;52(6):1066–1093. doi: 10.1152/jn.1984.52.6.1066. [DOI] [PubMed] [Google Scholar]
- Dykes R. W., Rasmusson D. D., Hoeltzell P. B. Organization of primary somatosensory cortex in the cat. J Neurophysiol. 1980 Jun;43(6):1527–1546. doi: 10.1152/jn.1980.43.6.1527. [DOI] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Gardner E. P., Costanzo R. M. Spatial integration of multiple-point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol. 1980 Feb;43(2):420–443. doi: 10.1152/jn.1980.43.2.420. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G., DeFelipe J., Schmechel D., Brandon C., Emson P. C. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6526–6530. doi: 10.1073/pnas.81.20.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13(3):277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Hicks T. P. Antagonism of synaptic transmission in vivo: contributions of microiontophoresis. Brain Behav Evol. 1983;22(1):1–12. doi: 10.1159/000121502. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Dykes R. W. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res. 1983 Sep 5;274(1):160–164. doi: 10.1016/0006-8993(83)90533-4. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Metherate R., Landry P., Dykes R. W. Bicuculline-induced alterations of response properties in functionally identified ventroposterior thalamic neurones. Exp Brain Res. 1986;63(2):248–264. doi: 10.1007/BF00236843. [DOI] [PubMed] [Google Scholar]
- Hicks T. P. The history and development of microiontophoresis in experimental neurobiology. Prog Neurobiol. 1984;22(3):185–240. doi: 10.1016/0301-0082(84)90019-4. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Hendry S. H., Jones E. G., Vaughn J. E. Morphological diversity of immunocytochemically identified GABA neurons in the monkey sensory-motor cortex. J Neurocytol. 1983 Aug;12(4):617–638. doi: 10.1007/BF01181527. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Hailstone M. H., Freeman C. G. Baclofen: stereoselective inhibition of excitant amino acid release. J Pharm Pharmacol. 1980 Mar;32(3):230–231. doi: 10.1111/j.2042-7158.1980.tb12902.x. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. An electron microscopic study of the laminar pattern and mode of termination of afferent fibre pathways in the somatic sensory cortex of the cat. Philos Trans R Soc Lond B Biol Sci. 1970 Jan 29;257(812):45–62. doi: 10.1098/rstb.1970.0007. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Hicks T. P. Baclofen and gamma-aminobutyric acid differentially suppress the cutaneous responsiveness of primary somatosensory cortical neurones. Brain Res. 1988 Mar 8;443(1-2):360–366. doi: 10.1016/0006-8993(88)91634-4. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka J. I., Jang E. K., Hicks T. P. Benzodiazepine receptor involvement in the control of receptive field size and responsiveness in primary somatosensory cortex. Brain Res. 1986 Jun 18;376(1):194–198. doi: 10.1016/0006-8993(86)90917-0. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release. Can J Physiol Pharmacol. 1978 Feb;56(1):150–154. doi: 10.1139/y78-019. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Freund T. F., Kisvárday Z. F. Different types of 3H-GABA accumulating neurons in the visual cortex of the rat. Characterization by combined autoradiography and Golgi impregnation. Exp Brain Res. 1984;54(1):45–56. doi: 10.1007/BF00235817. [DOI] [PubMed] [Google Scholar]
- Sretavan D., Dykes R. W. The organization of two cutaneous submodalities in the forearm region of area 3b of cat somatosensory cortex. J Comp Neurol. 1983 Feb 1;213(4):381–398. doi: 10.1002/cne.902130403. [DOI] [PubMed] [Google Scholar]
- Valverde F. Aspects of cortical organization related to the geometry of neurons with intra-cortical axons. J Neurocytol. 1976 Oct;5(8):509–529. doi: 10.1007/BF01175566. [DOI] [PubMed] [Google Scholar]