Abstract
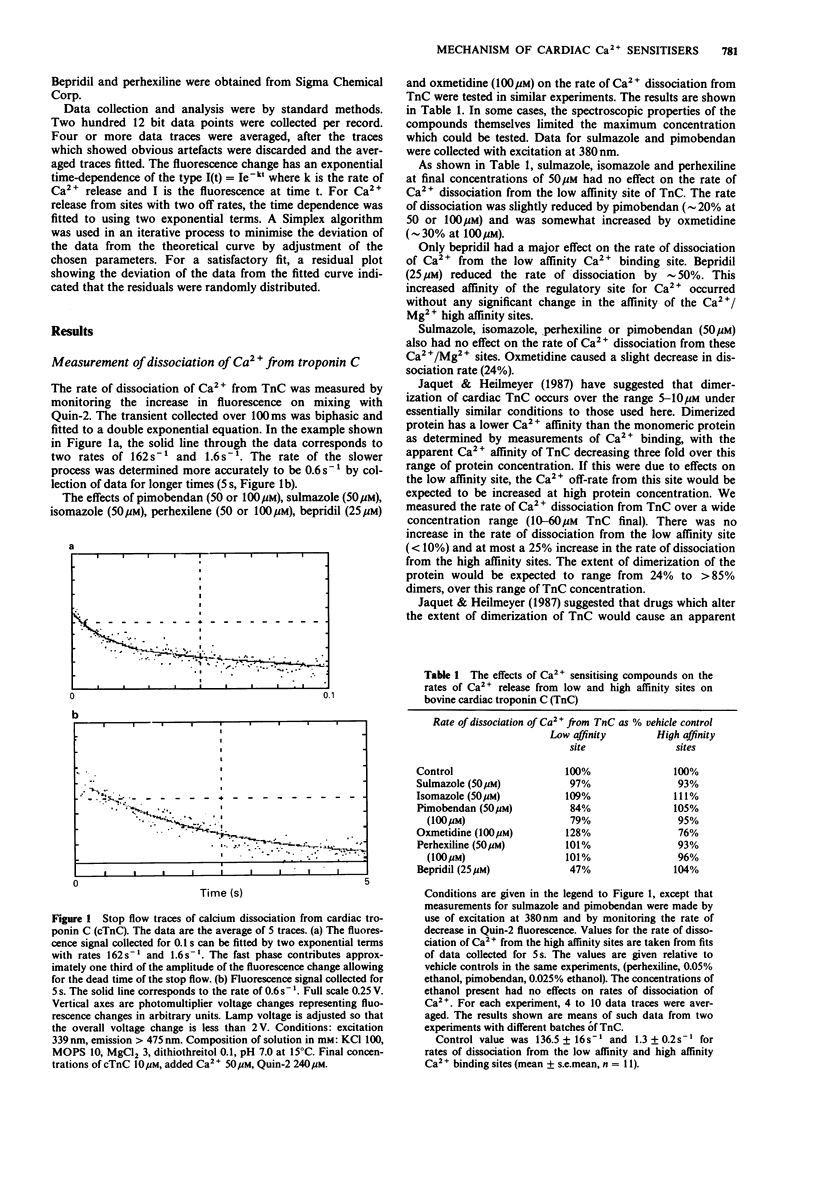
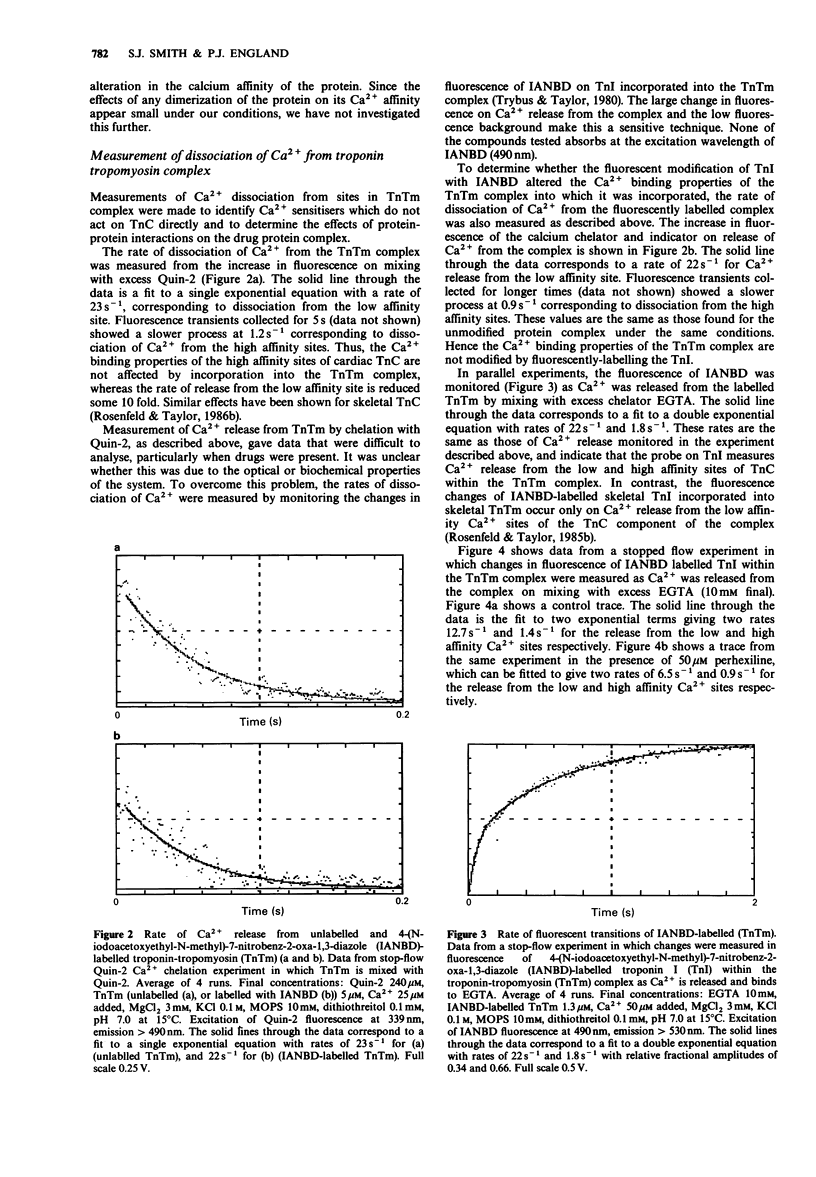
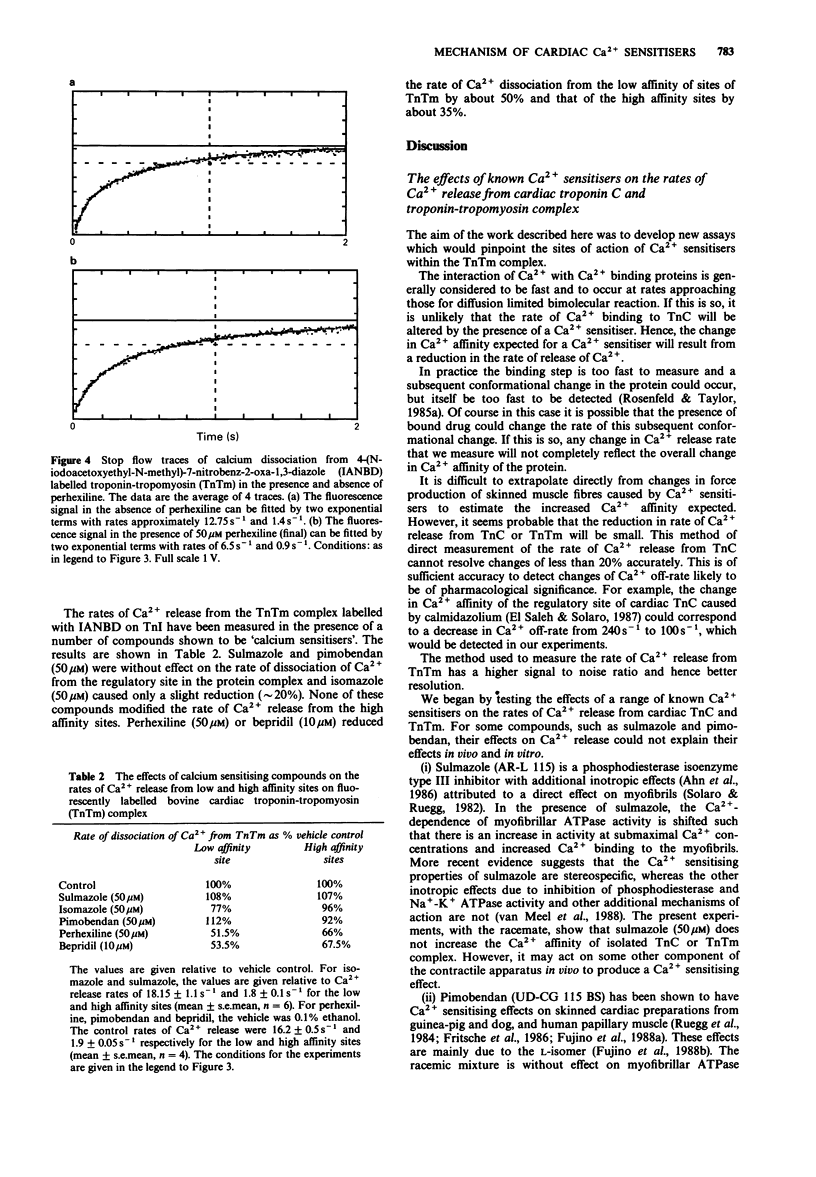
1. The calcium sensitivity of force production of cardiac muscle fibres is altered by certain drugs. The sites of action of three such compounds (pimobendan, sulmazole, isomazole) within the myofibril have been investigated. Calmodulin antagonists, perhexilene and bepridil, which have been shown to alter the calcium dependence of myofibrillar ATPase activity and oxmetidine, an H2-receptor antagonist which binds to calmodulin, were also studied. 2. The rates of dissociation of calcium from both the regulatory and high affinity sites on bovine isolated cardiac troponin C (cTnC) were measured in a stopped-flow fluorimeter. The rates of dissociation were found to be 136.5 +/- 16 s-1 and 1.3 +/- 0.20 s-1 (mean +/- s.e.mean, n = 11 determinations; conditions: 100 mM KCl, 10 mM MOPS, 3 mM MgCl2, 0.1 mM dithriothreitol, pH 7.0, 15 degrees C). Sulmazole, isomazole and perhexiline (final concentration of 50 microM) had no effect on the rate of Ca2+ dissociation from the regulatory Ca2+ site, indicating that these compounds do not act on cTnC directly. 3. The rate of dissociation of Ca2+ from the regulatory site was slightly reduced (approximately 20%) by pimobendan (50 and 100 microM) and was somewhat increased by oxmetidine (28% at 100 microM). 4. Bepridil (25 microM) reduced the rate of dissociation by 50%, indicating a direct effect of bepridil on TnC. 5. Sulmazole, isomazole, perhexiline, pimobendan (50 microM) and bepridil (25 microM) were without effect on the rate of dissociation of Ca2+ from the high affinity Ca2+/Mg2+ sites. Oxmetidine caused 24% decrease in the rate of Ca2+ dissociation from these sites.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Ahn H. S., Eardley D., Watkins R., Prioli N. Effects of several newer cardiotonic drugs on cardiac cyclic AMP metabolism. Biochem Pharmacol. 1986 Apr 1;35(7):1113–1121. doi: 10.1016/0006-2952(86)90147-4. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fujino K., Sperelakis N., Solaro R. J. Differential effects of d- and l-pimobendan on cardiac myofilament calcium sensitivity. J Pharmacol Exp Ther. 1988 Nov;247(2):519–523. [PubMed] [Google Scholar]
- Fujino K., Sperelakis N., Solaro R. J. Sensitization of dog and guinea pig heart myofilaments to Ca2+ activation and the inotropic effect of pimobendan: comparison with milrinone. Circ Res. 1988 Nov;63(5):911–922. doi: 10.1161/01.res.63.5.911. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Hill T. L. Two elementary models for the regulation of skeletal muscle contraction by calcium. Biophys J. 1983 Dec;44(3):383–396. doi: 10.1016/S0006-3495(83)84312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet K., Heilmeyer L. M., Jr Influence of association and of positive inotropic drugs on calcium binding to cardiac troponin C. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1390–1396. doi: 10.1016/0006-291x(87)91592-0. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Charlton S. C., Potter J. D. A fluorescence stopped flow analysis of Ca2+ exchange with troponin C. J Biol Chem. 1979 May 10;254(9):3497–3502. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leszyk J., Dumaswala R., Potter J. D., Collins J. H. Amino acid sequence of bovine cardiac troponin I. Biochemistry. 1988 Apr 19;27(8):2821–2827. doi: 10.1021/bi00408a024. [DOI] [PubMed] [Google Scholar]
- Lues I., Siegel R., Harting J. Effect of isomazole on the responsiveness to calcium of the contractile elements in skinned cardiac muscle fibres of various species. Eur J Pharmacol. 1988 Jan 27;146(1):145–153. doi: 10.1016/0014-2999(88)90496-7. [DOI] [PubMed] [Google Scholar]
- Pan B. S., Solaro R. J. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J Biol Chem. 1987 Jun 5;262(16):7839–7849. [PubMed] [Google Scholar]
- Reid D. G. Protein binding properties of some histamine H2 antagonists: a 1H nuclear magnetic resonance study of antihistamine-calmodulin interactions. Biochim Biophys Acta. 1986 Apr 8;886(1):18–25. doi: 10.1016/0167-4889(86)90206-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. Kinetic studies of calcium and magnesium binding to troponin C. J Biol Chem. 1985 Jan 10;260(1):242–251. [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. Kinetic studies of calcium binding to regulatory complexes from skeletal muscle. J Biol Chem. 1985 Jan 10;260(1):252–261. [PubMed] [Google Scholar]
- Rüegg J. C., Pfitzer G., Eubler D., Zeugner C. Effect on contractility of skinned fibres from mammalian heart and smooth muscle by a new benzimidazole derivative, 4,5-dihydro-6-[2-(4-methoxyphenyl)-1H-benzimidazol-5-yl]-5-methy l-3(2H )- pyridazinone. Arzneimittelforschung. 1984;34(12):1736–1738. [PubMed] [Google Scholar]
- Silver P. J., Pinto P. B., Dachiw J. Modulation of vascular and cardiac contractile protein regulatory mechanisms by calmodulin inhibitors and related compounds. Biochem Pharmacol. 1986 Aug 1;35(15):2545–2551. doi: 10.1016/0006-2952(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Bousquet P., Johnson J. D. Stimulation of cardiac myofilament force, ATPase activity and troponin C Ca++ binding by bepridil. J Pharmacol Exp Ther. 1986 Aug;238(2):502–507. [PubMed] [Google Scholar]
- Solaro R. J., Rüegg J. C. Stimulation of Ca++ binding and ATPase activity of dog cardiac myofibrils by AR-L 115BS, a novel cardiotonic agent. Circ Res. 1982 Sep;51(3):290–294. doi: 10.1161/01.res.51.3.290. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Buss J. E. Phosphorylation of cardiac troponin by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Feb 10;252(3):851–857. [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel B., Hauel N. New cardiotonic agents--a promising approach for treatment of heart failure. Trends Pharmacol Sci. 1988 May;9(5):166–170. doi: 10.1016/0165-6147(88)90031-4. [DOI] [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophys Biophys Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]
- van Eerd J. P., Takahashi K. The amino acid sequence of bovine cardiac tamponin-C. Comparison with rabbit skeletal troponin-C. Biochem Biophys Res Commun. 1975 May 5;64(1):122–127. doi: 10.1016/0006-291x(75)90227-2. [DOI] [PubMed] [Google Scholar]
- van Meel J. C., Zimmermann R., Diederen W., Erdman E., Mrwa U. Increase in calcium sensitivity of cardiac myofibrils contributes to the cardiotonic action of sulmazole. Biochem Pharmacol. 1988 Jan 15;37(2):213–220. doi: 10.1016/0006-2952(88)90720-4. [DOI] [PubMed] [Google Scholar]


