Abstract
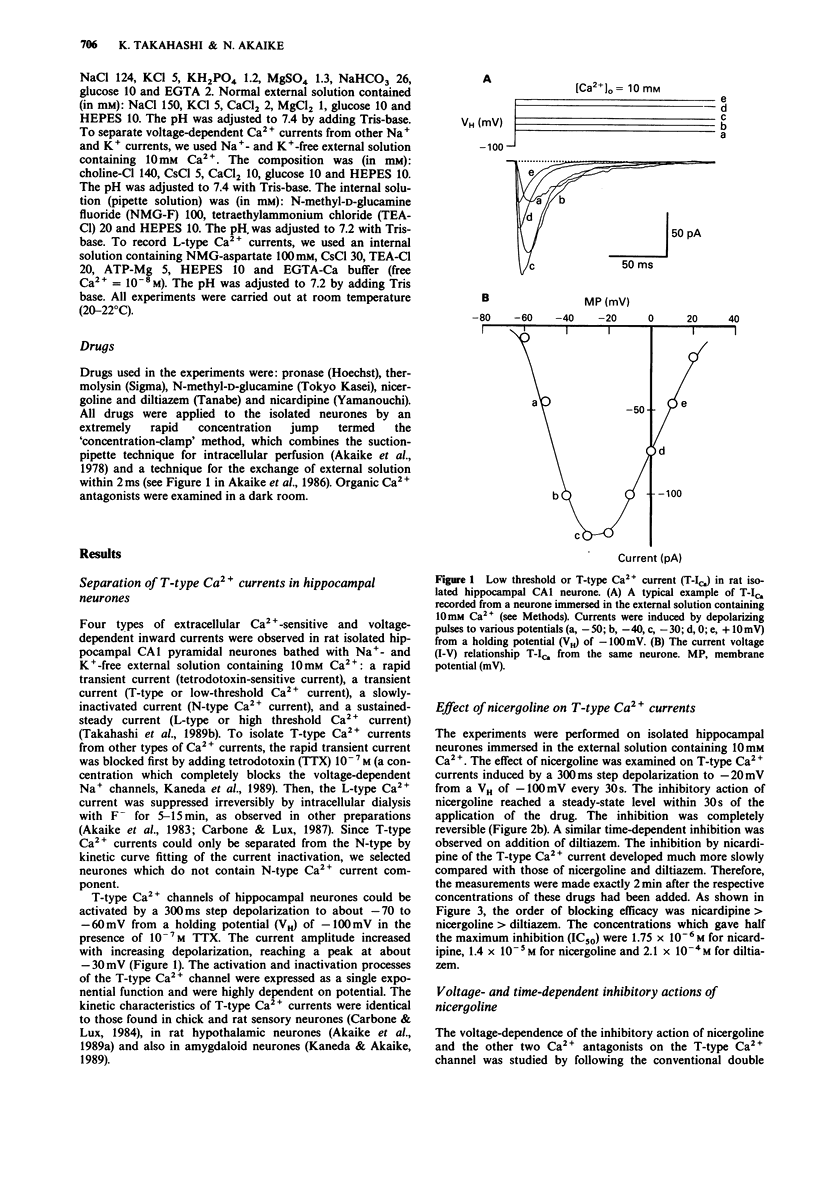
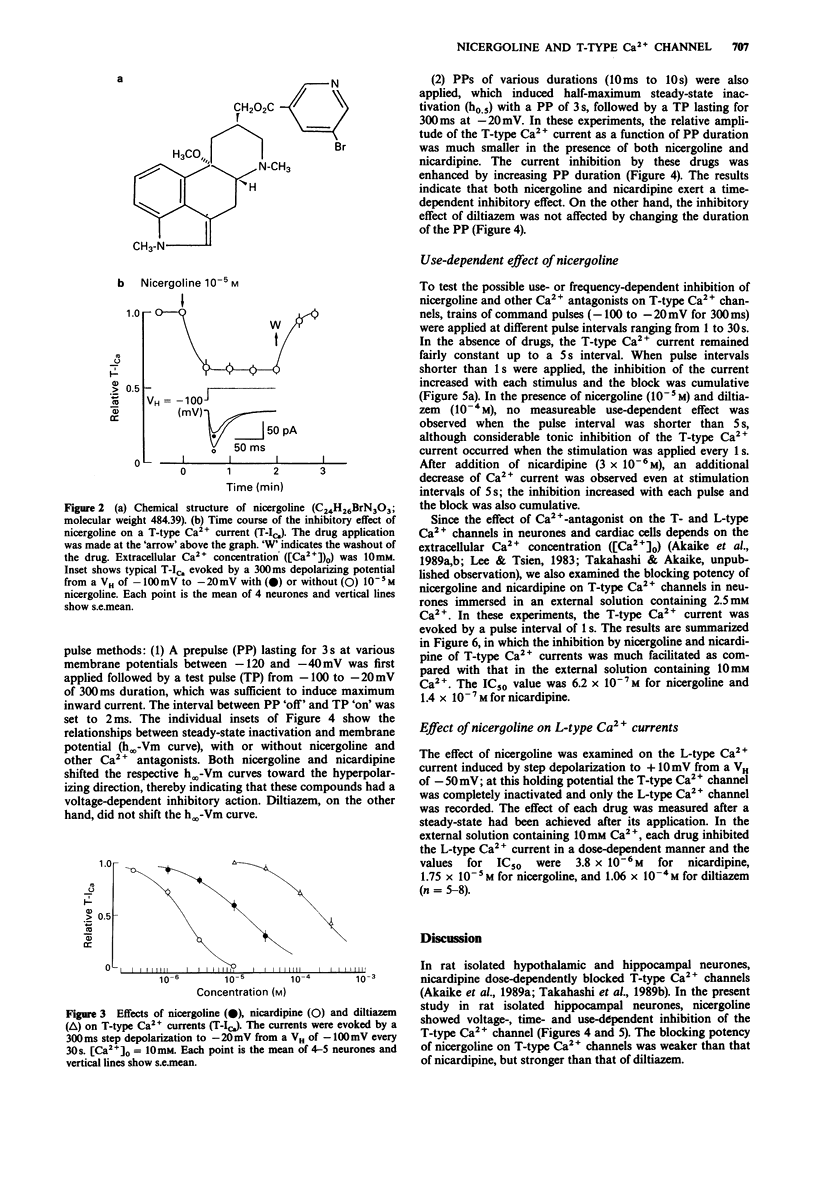
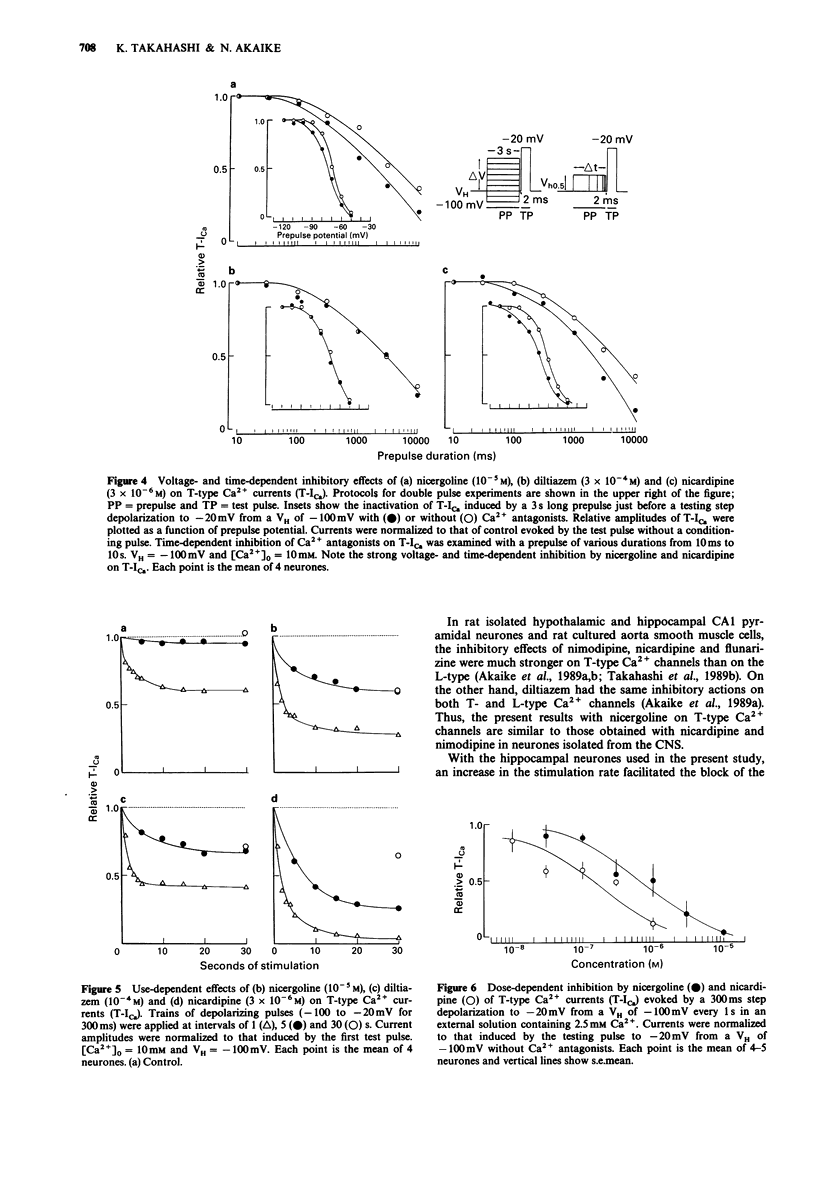
1. The effects of nicergoline on the T- and L-type Ca2+ currents in pyramidal cells freshly isolated from rat hippocampal CA1 region were investigated by use of a 'concentration-clamp' technique. The technique combines a suction-pipette technique, which allows intracellular perfusion under a single-electrode voltage-clamp, and rapid exchange of extracellular solution within 2 ms. 2. T-type Ca2+ currents were evoked by step depolarizations from a holding potential of -100 mV to potentials more positive than -70 to -60 mV, and reached a peak at about -30 mV in the current-voltage relationship. Activation and inactivation of T-type Ca2+ currents were highly potential-dependent. 3. Nicergoline and other Ca2+ antagonists dose-dependently blocked the T-type Ca2+ channel with an order of potency nicardipine greater than nicergoline greater than diltiazem. 4. The L-type Ca2+ channel was also blocked in the order nicardipine greater than nicergoline greater than diltiazem, although the T-type Ca2+ channel was more sensitive to nicergoline. 5. The inhibitory effects of nicergoline and nicardipine on the T-type Ca2+ current were voltage-, time-, and use-dependent, and the inhibition increased with a decrease in the external Ca2+ concentration. Diltiazem showed only a use-dependent block.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Kanaide H., Kuga T., Nakamura M., Sadoshima J., Tomoike H. Low-voltage-activated calcium current in rat aorta smooth muscle cells in primary culture. J Physiol. 1989 Sep;416:141–160. doi: 10.1113/jphysiol.1989.sp017754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Kostyuk P. G., Osipchuk Y. V. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol. 1989 May;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T., Nuglisch J., Sauer D., Bielenberg G. W., Mennel H. D., Rossberg C., Krieglstein J. Effects of flunarizine on postischemic blood flow, energy metabolism and neuronal damage in the rat brain. Eur J Pharmacol. 1988 Dec 13;158(3):271–274. doi: 10.1016/0014-2999(88)90078-7. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Courtney K. R. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975 Nov;195(2):225–236. [PubMed] [Google Scholar]
- Cullen J. P., Aldrete J. A., Jankovsky L., Romo-Salas F. Protective action of phenytoin in cerebral ischemia. Anesth Analg. 1979 May-Jun;58(3):165–169. [PubMed] [Google Scholar]
- Heitz C., Descombes J. J., Miller R. C., Stoclet J. C. Alpha-adrenoceptor antagonistic and calcium antagonistic effects of nicergoline in the rat isolated aorta. Eur J Pharmacol. 1986 Apr 16;123(2):279–285. doi: 10.1016/0014-2999(86)90669-2. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Akaike N. The low-threshold Ca current in isolated amygdaloid neurons in the rat. Brain Res. 1989 Sep 11;497(1):187–190. doi: 10.1016/0006-8993(89)90987-6. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Oomura Y., Ishibashi O., Akaike N. Permeability to various cations of the voltage-dependent sodium channel of isolated rat hippocampal pyramidal neurons. Neurosci Lett. 1988 Jun 7;88(3):253–256. doi: 10.1016/0304-3940(88)90219-4. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Shintomi K., Itakura T., Yoshimoto K., Ogawa Y., Fukushima T., Matsuoka Y. [Pharmacological study of nicergoline. (II). Protective effect on ischemic brain damages in animals]. Nihon Yakurigaku Zasshi. 1986 Apr;87(4):427–434. doi: 10.1254/fpj.87.427. [DOI] [PubMed] [Google Scholar]
- Steen P. A., Gisvold S. E., Milde J. H., Newberg L. A., Scheithauer B. W., Lanier W. L., Michenfelder J. D. Nimodipine improves outcome when given after complete cerebral ischemia in primates. Anesthesiology. 1985 Apr;62(4):406–414. doi: 10.1097/00000542-198504000-00007. [DOI] [PubMed] [Google Scholar]
- Steen P. A., Newberg L. A., Milde J. H., Michenfelder J. D. Nimodipine improves cerebral blood flow and neurologic recovery after complete cerebral ischemia in the dog. J Cereb Blood Flow Metab. 1983 Mar;3(1):38–43. doi: 10.1038/jcbfm.1983.4. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tateishi N., Kaneda M., Akaike N. Comparison of low-threshold Ca2+ currents in the hippocampal CA1 neurons among the newborn, adult and aged rats. Neurosci Lett. 1989 Aug 14;103(1):29–33. doi: 10.1016/0304-3940(89)90480-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Wakamori M., Akaike N. Hippocampal CA1 pyramidal cells of rats have four voltage-dependent calcium conductances. Neurosci Lett. 1989 Sep 25;104(1-2):229–234. doi: 10.1016/0304-3940(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Yaari Y., Hamon B., Lux H. D. Development of two types of calcium channels in cultured mammalian hippocampal neurons. Science. 1987 Feb 6;235(4789):680–682. doi: 10.1126/science.2433765. [DOI] [PubMed] [Google Scholar]


