Abstract
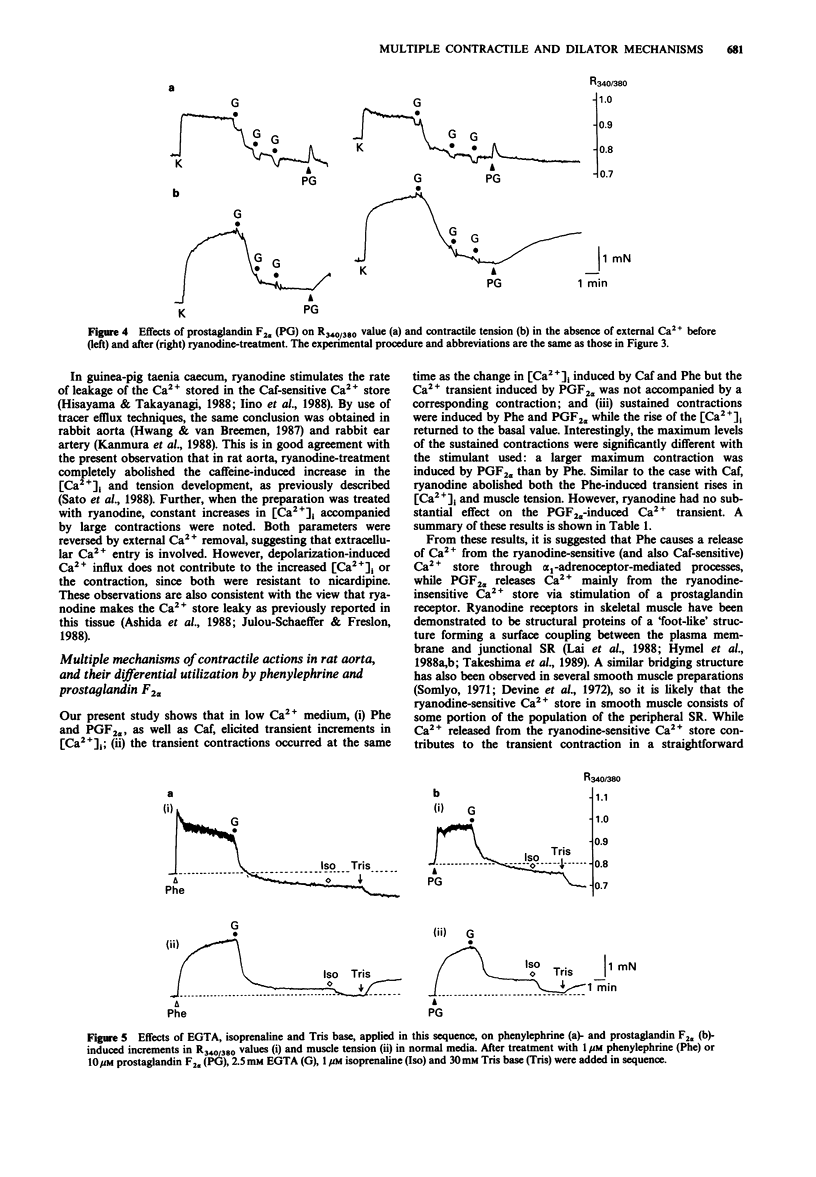
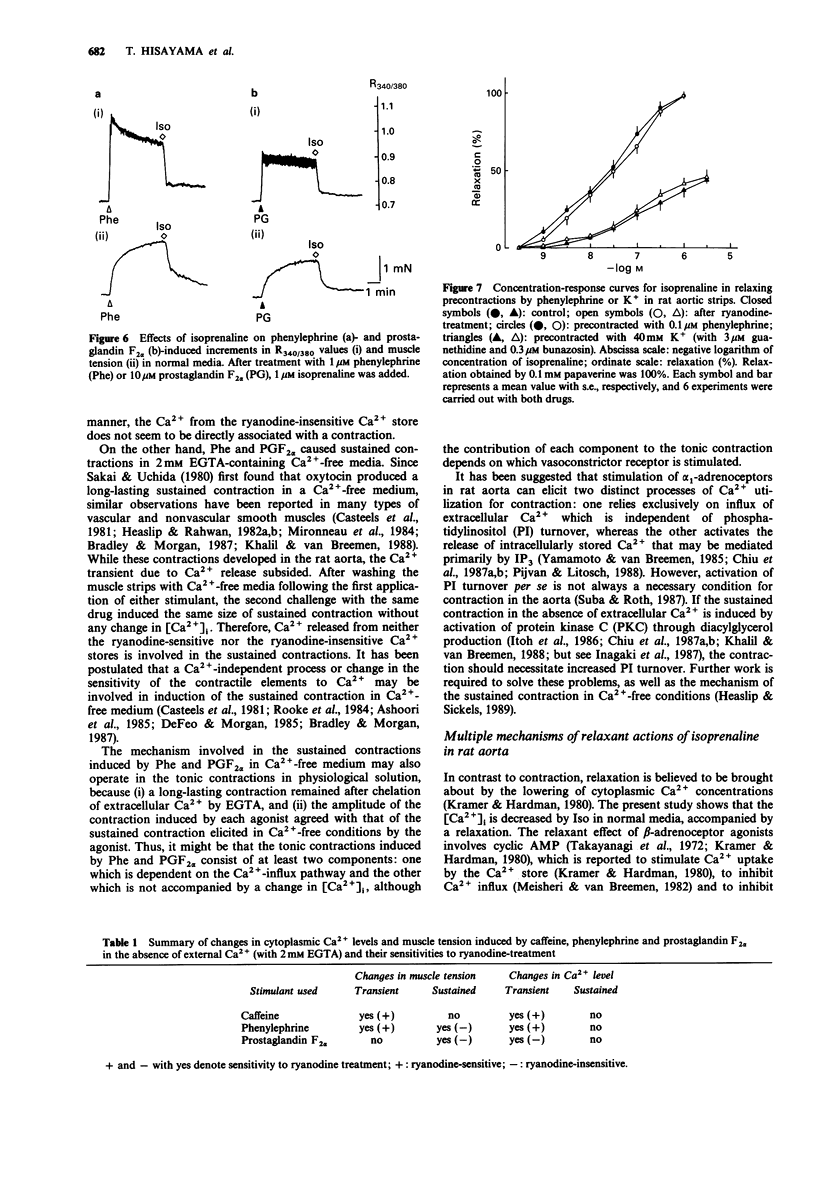
1. The effects of ryanodine on changes in cytoplasmic Ca2+ level ([Ca2+]i) and muscle tension induced by maximum concentrations of phenylephrine (Phe; 1 microM), prostaglandin F2 alpha (PGF2 alpha, 10 microM), caffeine (Caf, 30 mM) and isoprenaline (Iso, 1 microM) were examined in rat aortic strips using fura-2. 2. In normal media, Phe and PGF2 alpha produced a phasic contraction, followed by a tonic one. Caf elicited only a transient contraction. When the preparation was treated with 10 microM ryanodine, an increase in [Ca2+]i was induced accompanied by a nicardipine (1 microM)-resistant contraction which was [Ca2+]o-dependent. 3. In Ca2(+)-free solution, the three stimulants elicited transient increases in [Ca2+]i. Transient contractions to Phe and Caf were accompanied by changes in [Ca2+]i. The transient increase in [Ca2+]i induced by PGF2 alpha was not accompanied by a corresponding contraction. 4. Sustained contractions were induced by Phe and PGF2 alpha in the absence of external Ca2+, while the increase in [Ca2+]i was reduced. A larger maximum contraction was induced by PGF alpha than by Phe. 5. Ryanodine abolished both the Caf- and Phe-induced [Ca2+]i transient increases and the corresponding contractions, but had no substantial effect on the PGF2 alpha-induced [Ca2+]i transient increase. Ryanodine had no influence on the sustained contractions induced by Phe and PGF2 alpha. 6. Iso relaxed both sustained contractions almost completely, without any detectable change in [Ca2+]i. Treatment of the preparation with ryanodine had no effect on the concentration-response curves for Iso in relaxing the 0.1 microM Phe- or 40 mM K(+)-induced precontraction.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

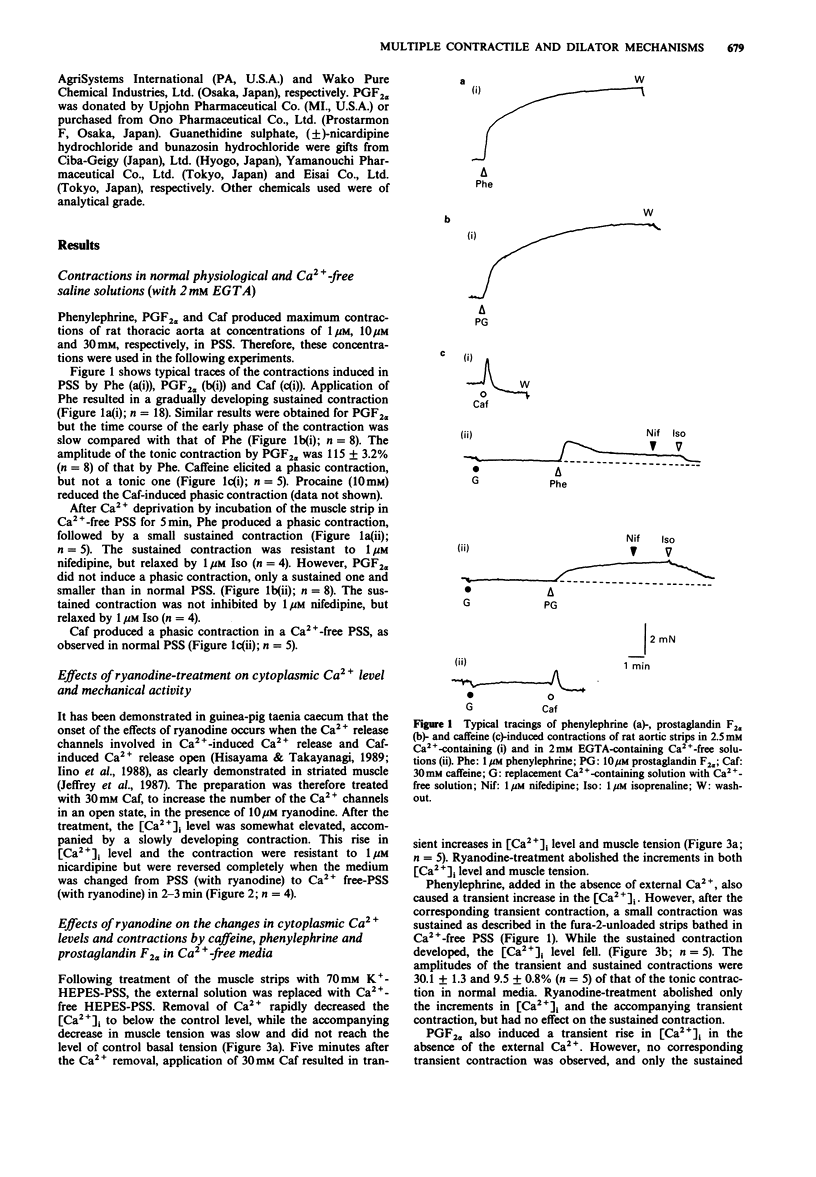
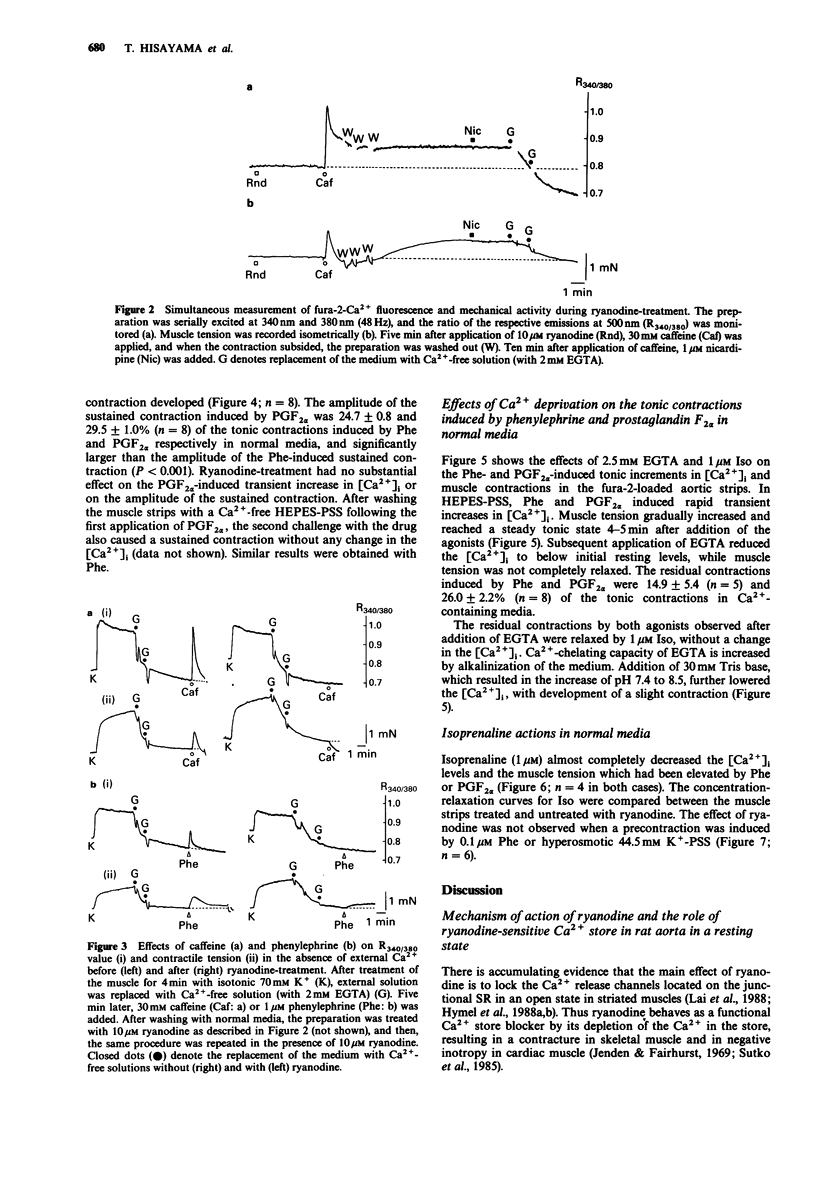


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Hathaway D. R. Role of calcium and cyclic adenosine 3':5' monophosphate in regulating smooth muscle contraction. Mechanisms of excitation-contraction coupling in smooth muscle. Am J Cardiol. 1979 Oct 22;44(5):783–787. doi: 10.1016/0002-9149(79)90197-8. [DOI] [PubMed] [Google Scholar]
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Ashida T., Schaeffer J., Goldman W. F., Wade J. B., Blaustein M. P. Role of sarcoplasmic reticulum in arterial contraction: comparison of ryanodines's effect in a conduit and a muscular artery. Circ Res. 1988 Apr;62(4):854–863. doi: 10.1161/01.res.62.4.854. [DOI] [PubMed] [Google Scholar]
- Ashoori F., Takai A., Tomita T. The response of non-pregnant rat myometrium to oxytocin in Ca-free solution. Br J Pharmacol. 1985 Jan;84(1):175–183. [PMC free article] [PubMed] [Google Scholar]
- Bradley A. B., Morgan K. G. Alterations in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. J Physiol. 1987 Apr;385:437–448. doi: 10.1113/jphysiol.1987.sp016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L., Suzuki H., Van Eldere J. Tension response and 45Ca release in vascular smooth muscle incubated in Ca-free solution. Pflugers Arch. 1981 Dec;392(2):139–145. doi: 10.1007/BF00581262. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Bozarth J. M., Forsythe M. S., Timmermans P. B. Ca++ utilization in the constriction of rat aorta to stimulation of protein kinase C by phorbol dibutyrate. J Pharmacol Exp Ther. 1987 Sep;242(3):934–939. [PubMed] [Google Scholar]
- Chiu A. T., Bozarth J. M., Timmermans P. B. Relationship between phosphatidylinositol turnover and Ca++ mobilization induced by alpha-1 adrenoceptor stimulation in the rat aorta. J Pharmacol Exp Ther. 1987 Jan;240(1):123–127. [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J Physiol. 1985 Dec;369:269–282. doi: 10.1113/jphysiol.1985.sp015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. F., Szentivanyi A. Stimulation of calcium uptake into aortic microsomes by cyclic AMP and cyclic AMP-dependent protein kinase. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jul;298(3):255–257. doi: 10.1007/BF00500896. [DOI] [PubMed] [Google Scholar]
- Heaslip R. J., Rahwan R. G. Evidence for mobilization of intracellular calcium during the contractile response of the rat aorta to U44069. Can J Physiol Pharmacol. 1982 May;60(5):743–746. doi: 10.1139/y82-103. [DOI] [PubMed] [Google Scholar]
- Heaslip R. J., Rahwan R. G. Evidence for the existence of two distinct pools of intracellular calcium in the rat aorta accessible to mobilization by norepinephrine. J Pharmacol Exp Ther. 1982 Apr;221(1):7–13. [PubMed] [Google Scholar]
- Heaslip R. J., Sickels B. D. Evidence that prostaglandins can contract the rat aorta via a novel protein kinase C-dependent mechanism. J Pharmacol Exp Ther. 1989 Jul;250(1):44–51. [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I. Effects of cyclic AMP and protein kinase on calcium uptake in a microsomal fraction from guinea pig taenia caecum. Biochem Pharmacol. 1983 Nov 1;32(21):3197–3203. doi: 10.1016/0006-2952(83)90204-6. [DOI] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I. Ryanodine: its possible mechanism of action in the caffeine-sensitive calcium store of smooth muscle. Pflugers Arch. 1988 Sep;412(4):376–381. doi: 10.1007/BF01907555. [DOI] [PubMed] [Google Scholar]
- Hwang K. S., van Breemen C. Ryanodine modulation of 45Ca efflux and tension in rabbit aortic smooth muscle. Pflugers Arch. 1987 Apr;408(4):343–350. doi: 10.1007/BF00581127. [DOI] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymel L., Schindler H., Inui M., Fleischer S. Reconstitution of purified cardiac muscle calcium release channel (ryanodine receptor) in planar bilayers. Biochem Biophys Res Commun. 1988 Apr 15;152(1):308–314. doi: 10.1016/s0006-291x(88)80715-0. [DOI] [PubMed] [Google Scholar]
- Iino M., Kobayashi T., Endo M. Use of ryanodine for functional removal of the calcium store in smooth muscle cells of the guinea-pig. Biochem Biophys Res Commun. 1988 Apr 15;152(1):417–422. doi: 10.1016/s0006-291x(88)80730-7. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Yokokura H., Itoh T., Kanmura Y., Kuriyama H., Hidaka H. Purified rabbit brain protein kinase C relaxes skinned vascular smooth muscle and phosphorylates myosin light chain. Arch Biochem Biophys. 1987 Apr;254(1):136–141. doi: 10.1016/0003-9861(87)90089-0. [DOI] [PubMed] [Google Scholar]
- Itoh T., Izumi H., Kuriyama H. Mechanisms of relaxation induced by activation of beta-adrenoceptors in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1982 May;326:475–493. doi: 10.1113/jphysiol.1982.sp014207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H., Sumimoto K. A phorbol ester has dual actions on the mechanical response in the rabbit mesenteric and porcine coronary arteries. J Physiol. 1986 Jun;375:515–534. doi: 10.1113/jphysiol.1986.sp016131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenden D. J., Fairhurst A. S. The pharmacology of ryanodine. Pharmacol Rev. 1969 Mar;21(1):1–25. [PubMed] [Google Scholar]
- Julou-Schaeffer G., Freslon J. L. Effects of ryanodine on tension development in rat aorta and mesenteric resistance vessels. Br J Pharmacol. 1988 Oct;95(2):605–613. doi: 10.1111/j.1476-5381.1988.tb11682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Raeymaekers L., Casteels R. Ryanodine reduces the amount of calcium in intracellular stores of smooth-muscle cells of the rabbit ear artery. Pflugers Arch. 1988 Dec;413(2):153–159. doi: 10.1007/BF00582525. [DOI] [PubMed] [Google Scholar]
- Khalil R. A., van Breemen C. Sustained contraction of vascular smooth muscle: calcium influx or C-kinase activation? J Pharmacol Exp Ther. 1988 Feb;244(2):537–542. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., van Breemen C. Effects of beta-adrenergic stimulation on calcium movements in rabbit aortic smooth muscle: relationship with cyclic AMP. J Physiol. 1982 Oct;331:429–441. doi: 10.1113/jphysiol.1982.sp014380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau C., Mironneau J., Savineau J. P. Maintained contractions of rat uterine smooth muscle incubated in a Ca2+-free solution. Br J Pharmacol. 1984 Jul;82(3):735–743. doi: 10.1111/j.1476-5381.1984.tb10813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijuan V., Litosch I. Norepinephrine stimulates the production of inositol trisphosphate and inositol tetrakisphosphate in rat aorta. Biochem Biophys Res Commun. 1988 Oct 14;156(1):240–245. doi: 10.1016/s0006-291x(88)80831-3. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem J. 1988 May 15;252(1):269–273. doi: 10.1042/bj2520269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke T. W., Rimele T. J., Vanhoutte P. M. Contractions of the canine coronary artery in calcium-free solution. Am J Physiol. 1984 Aug;247(2 Pt 2):H259–H263. doi: 10.1152/ajpheart.1984.247.2.H259. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sakai K., Uchida M. A calcium reversal phenomenon: differentiation of excitatory and inhibitory roles of Ca in uterine smooth muscle contraction. Jpn J Pharmacol. 1980 Jun;30(3):394–396. doi: 10.1254/jjp.30.394. [DOI] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Multiple effects of caffeine on contraction and cytosolic free Ca2+ levels in vascular smooth muscle of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):443–448. doi: 10.1007/BF00172125. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., North S. R. Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol. 1971 Dec;51(3):722–741. doi: 10.1083/jcb.51.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suba E. A., Roth B. L. Prostaglandins activate phosphoinositide metabolism in rat aorta. Eur J Pharmacol. 1987 Apr 29;136(3):325–332. doi: 10.1016/0014-2999(87)90305-0. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Ito K., Kenyon J. L. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc. 1985 Dec;44(15):2984–2988. [PubMed] [Google Scholar]
- Takayanagi I., Uchida M., Inatomi N., Tomiyama A., Takagi K. Intracellular cyclic adenosine 3', 5'-monophosphate and relaxing effects of isoprenaline and papaverine on smooth muscle of intestine. Jpn J Pharmacol. 1972 Dec;22(6):869–871. doi: 10.1254/jjp.22.869. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]


