Abstract
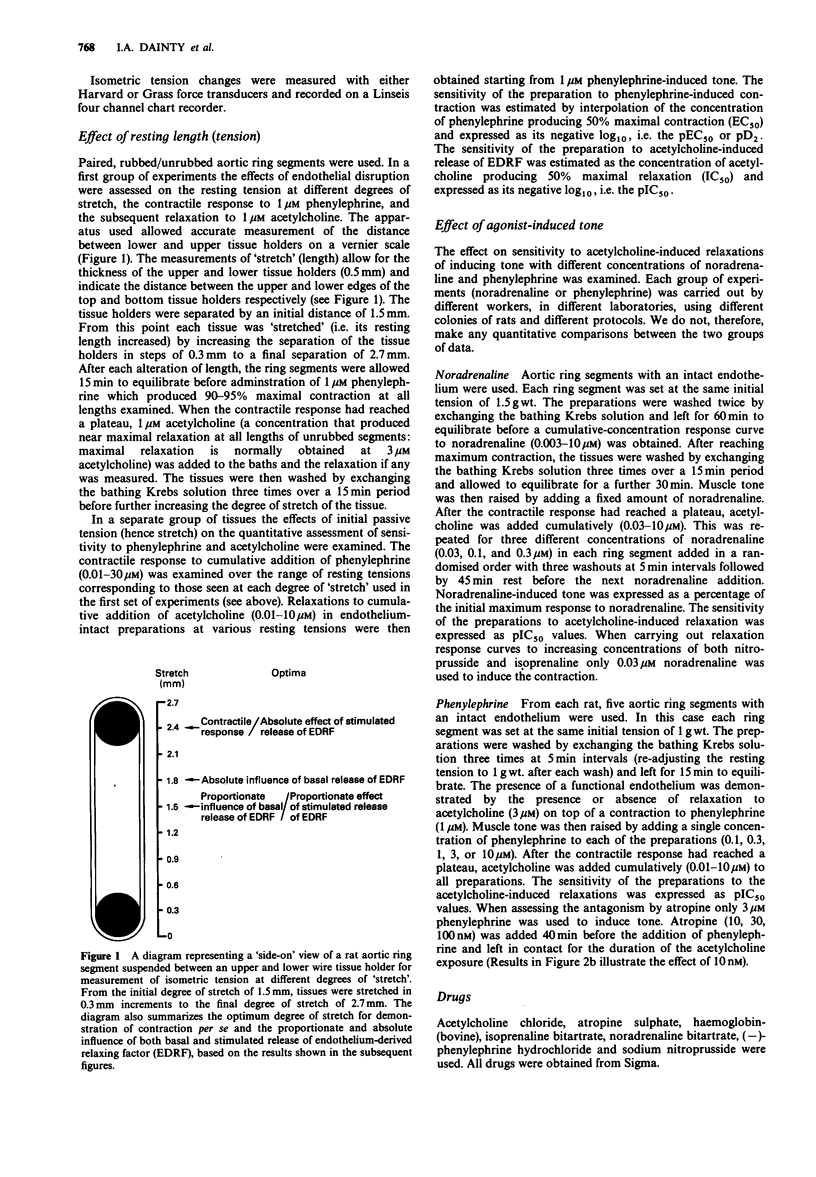
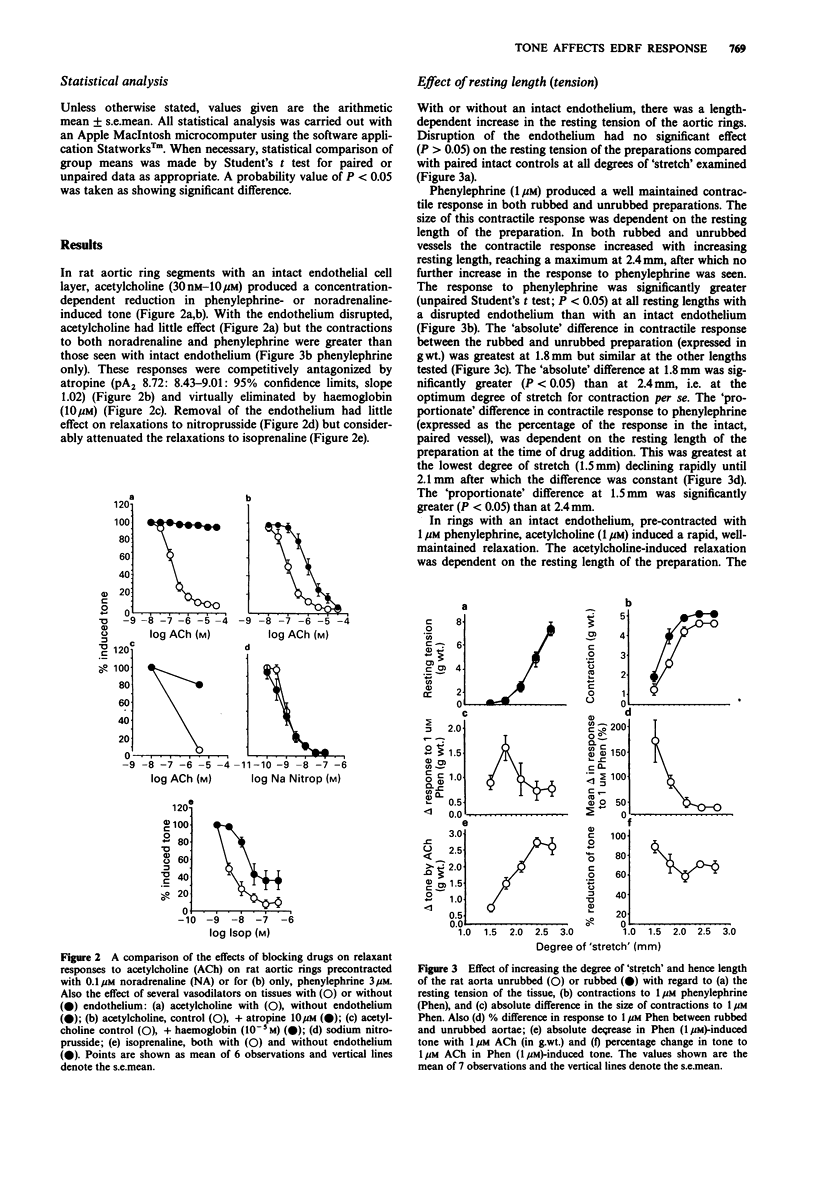
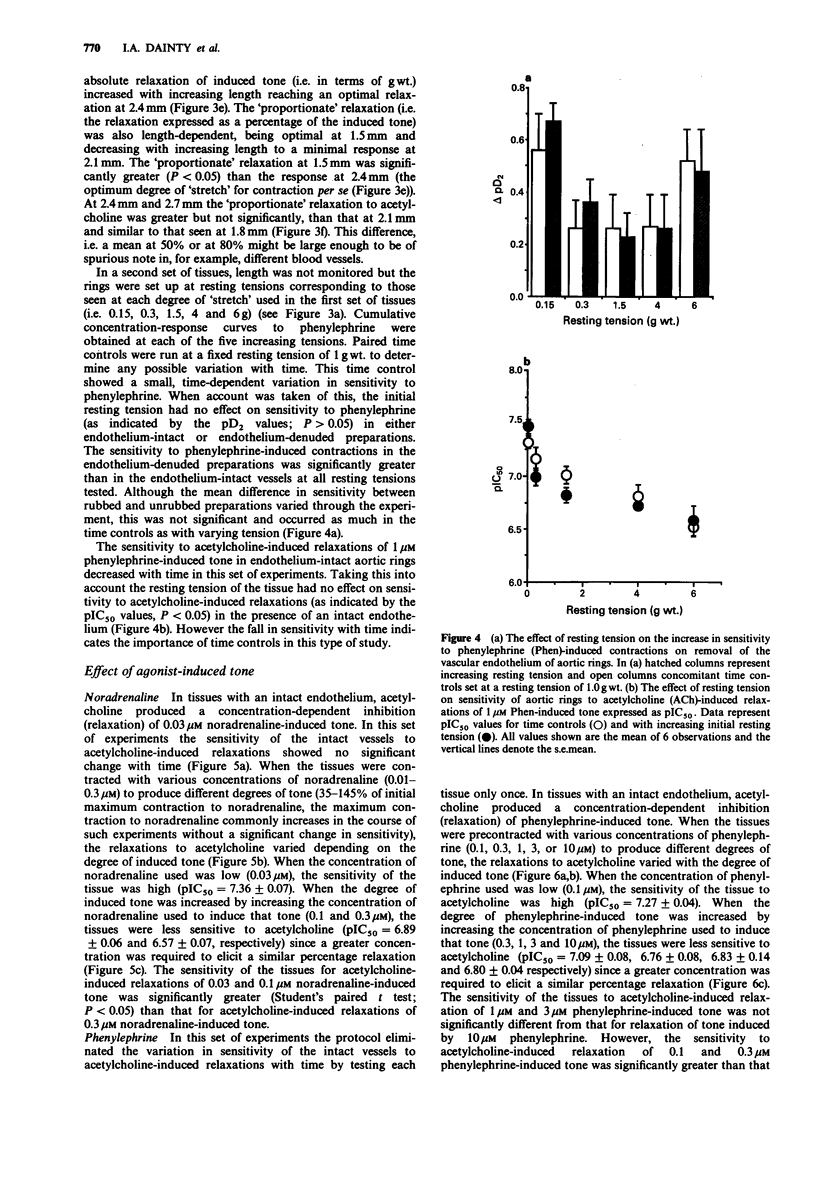
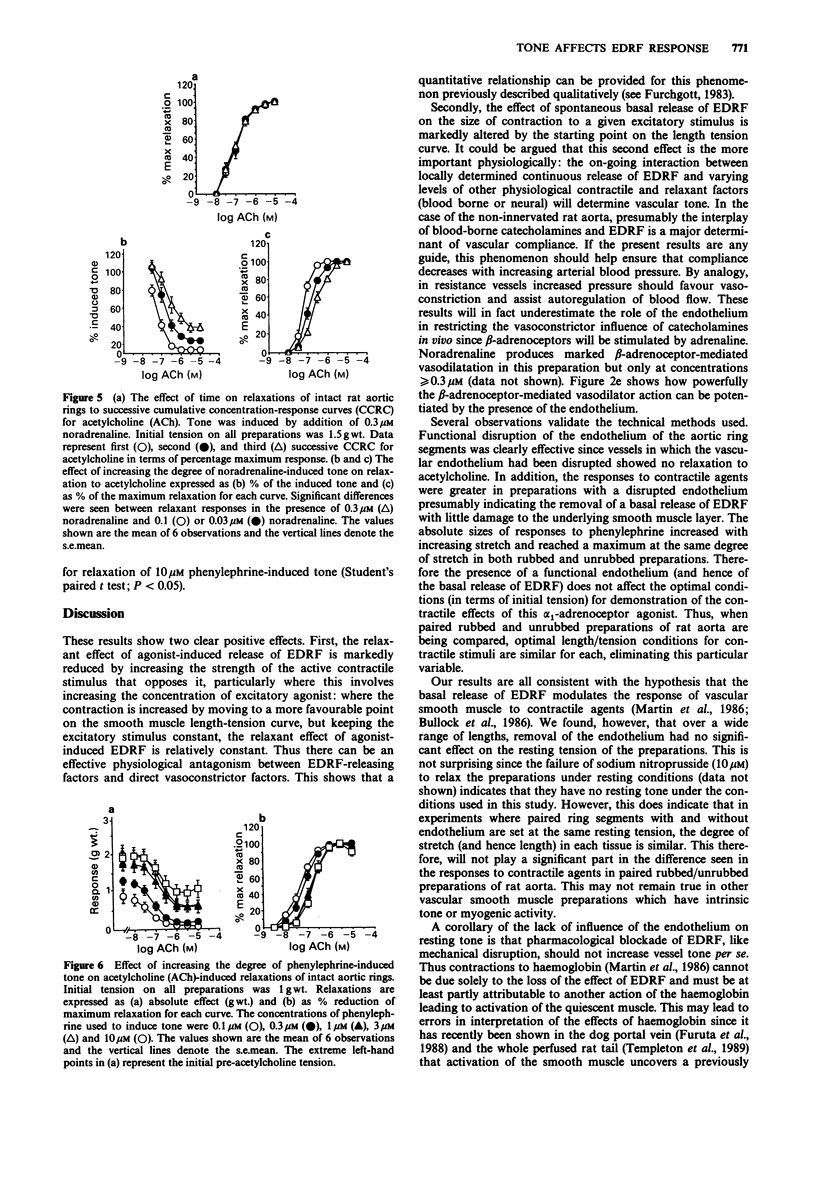
1. The effects of initial stretch and degree of agonist-induced tone on acetylcholine-induced relaxations were examined in rings of rat isolated aorta. The relaxation to acetylcholine was antagonized by atropine and almost completely abolished by haemoglobin. Relaxation to sodium nitroprusside was similar in rings with an intact or disrupted endothelium but that to isoprenaline was greater in intact preparations. 2. In preparations with either an intact or disrupted endothelium there was a similar length-dependent increase in the resting tension of the aortic rings. The size of the contractile response to phenylephrine (1 microM) was dependent on the initial length (and hence degree of stretch) of the preparation in both rubbed and unrubbed tissues. The absolute difference in contractile response between rubbed and unrubbed was greatest at 1.8 mm and less at the other lengths tested, including the optimum degree of stretch for contraction i.e. 2.4 mm. 3. The absolute acetylcholine-induced relaxation (only seen in rings with an intact endothelium) was dependent on the initial length (and hence degree of stretch) of the preparation and was maximum at 2.4 mm. The proportionate relaxation (i.e. expressed as a percentage of induced tone) was also length-dependent being optimal at 1.5 mm. 4. The sensitivity of the vessels to acetylcholine varied depending on the level of agonist-induced tone. When tone was low, acetylcholine sensitivity was high (at [NA] 0.03 microM: pIC50 = 7.36 +/- 0.07), when the concentration of noradrenaline was increased the tone increased and the acetylcholine sensitivity was low (at [NA] 0.3 microM: pIC50 = 6.57 +/- 0.07).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Cocks T. M., Satoh K. Alpha 2-adrenoceptors and endothelium-dependent relaxation in canine large arteries. Br J Pharmacol. 1986 Aug;88(4):767–777. doi: 10.1111/j.1476-5381.1986.tb16249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock G. R., Taylor S. G., Weston A. H. Influence of the vascular endothelium on agonist-induced contractions and relaxations in rat aorta. Br J Pharmacol. 1986 Dec;89(4):819–830. doi: 10.1111/j.1476-5381.1986.tb11187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Chappell S. P., Griffith T. M., Lewis M. J., Henderson A. H. Differences in basal endothelium-derived relaxing factor activity in different artery types. J Cardiovasc Pharmacol. 1986 Nov-Dec;8(6):1158–1162. doi: 10.1097/00005344-198611000-00010. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furuta T. Precontraction-induced contractile response of isolated canine portal vein to alpha-2 adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 1988 May;337(5):525–530. doi: 10.1007/BF00182726. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Sjöblom N. Distension-dependent changes in noradrenaline sensitivity in small arteries from the rat. Acta Physiol Scand. 1985 Nov;125(3):429–435. doi: 10.1111/j.1748-1716.1985.tb07739.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Templeton A. G., Macmillan J., McGrath J. C., Storey N. D., Wilson V. G. Evidence for prazosin-resistant, rauwolscine-sensitive alpha-adrenoceptors mediating contractions in the isolated vascular bed of the rat tail. Br J Pharmacol. 1989 Jun;97(2):563–571. doi: 10.1111/j.1476-5381.1989.tb11986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P. M. Vascular physiology: the end of the quest? Nature. 1987 Jun 11;327(6122):459–460. doi: 10.1038/327459a0. [DOI] [PubMed] [Google Scholar]


