Abstract
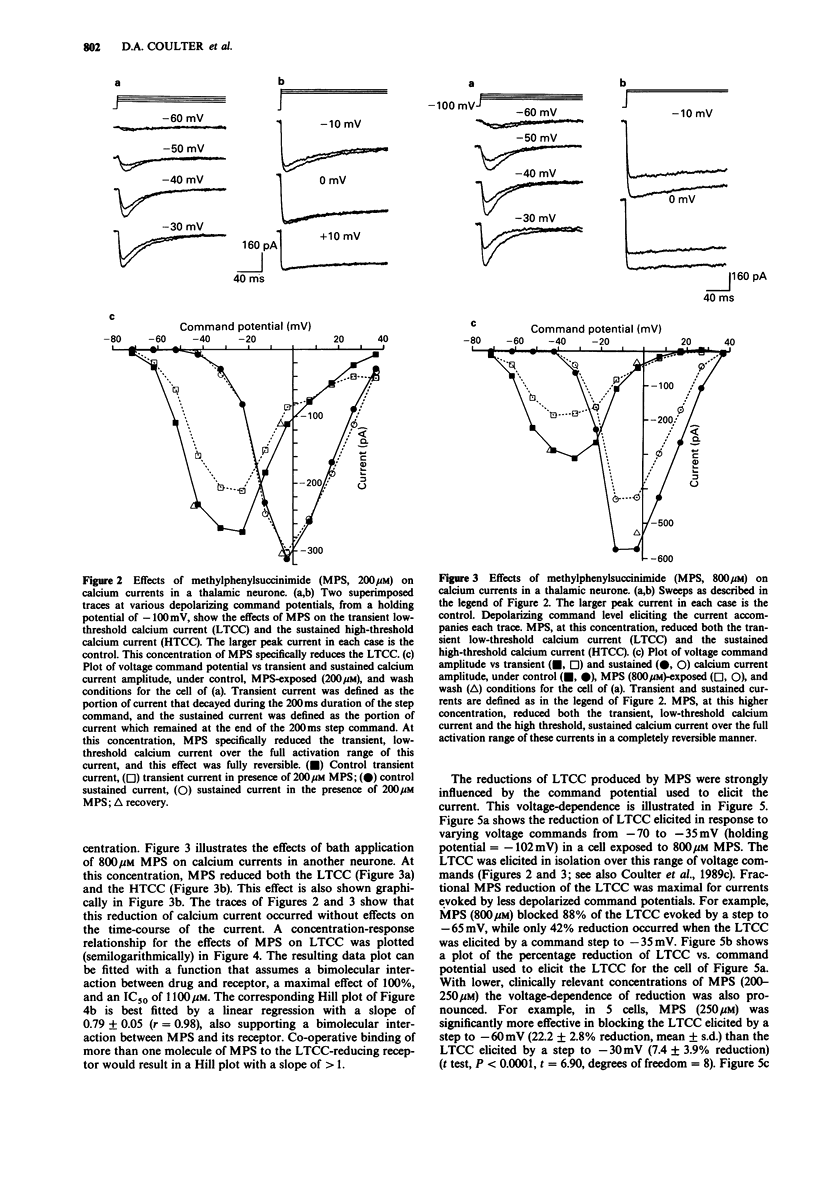
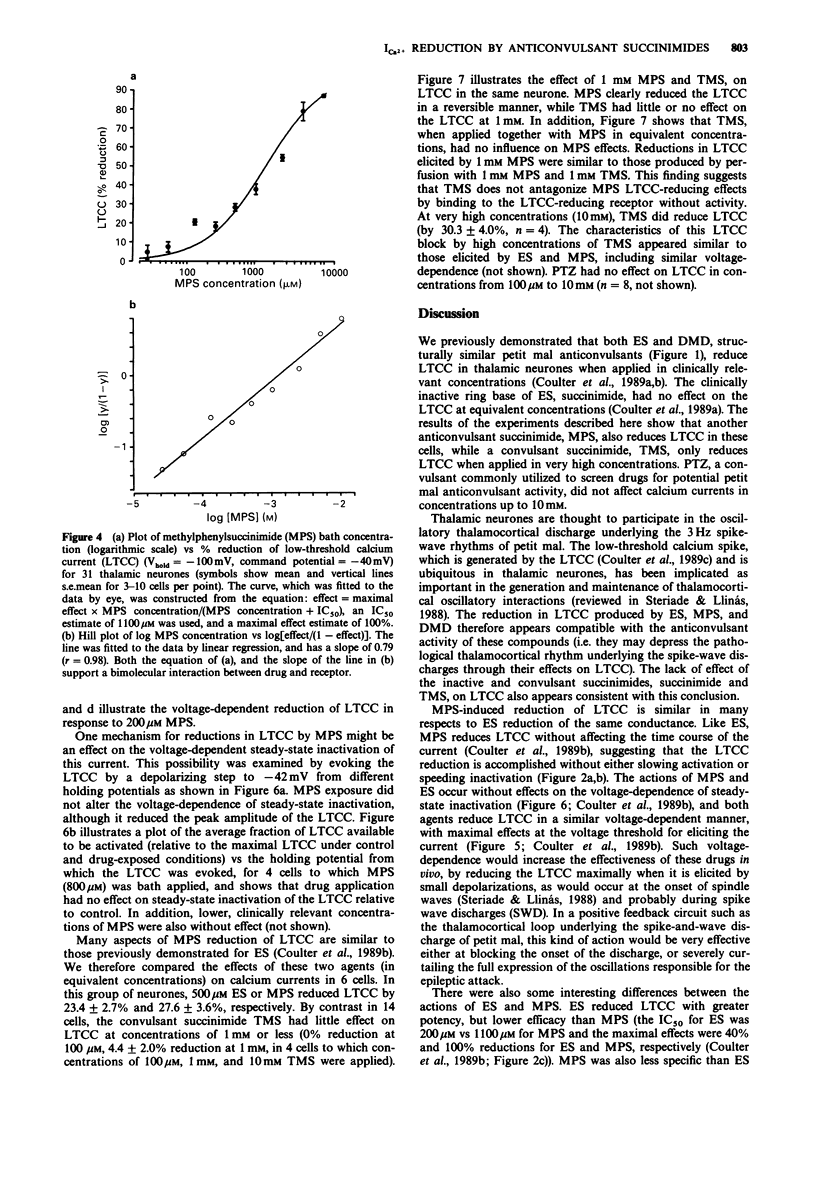
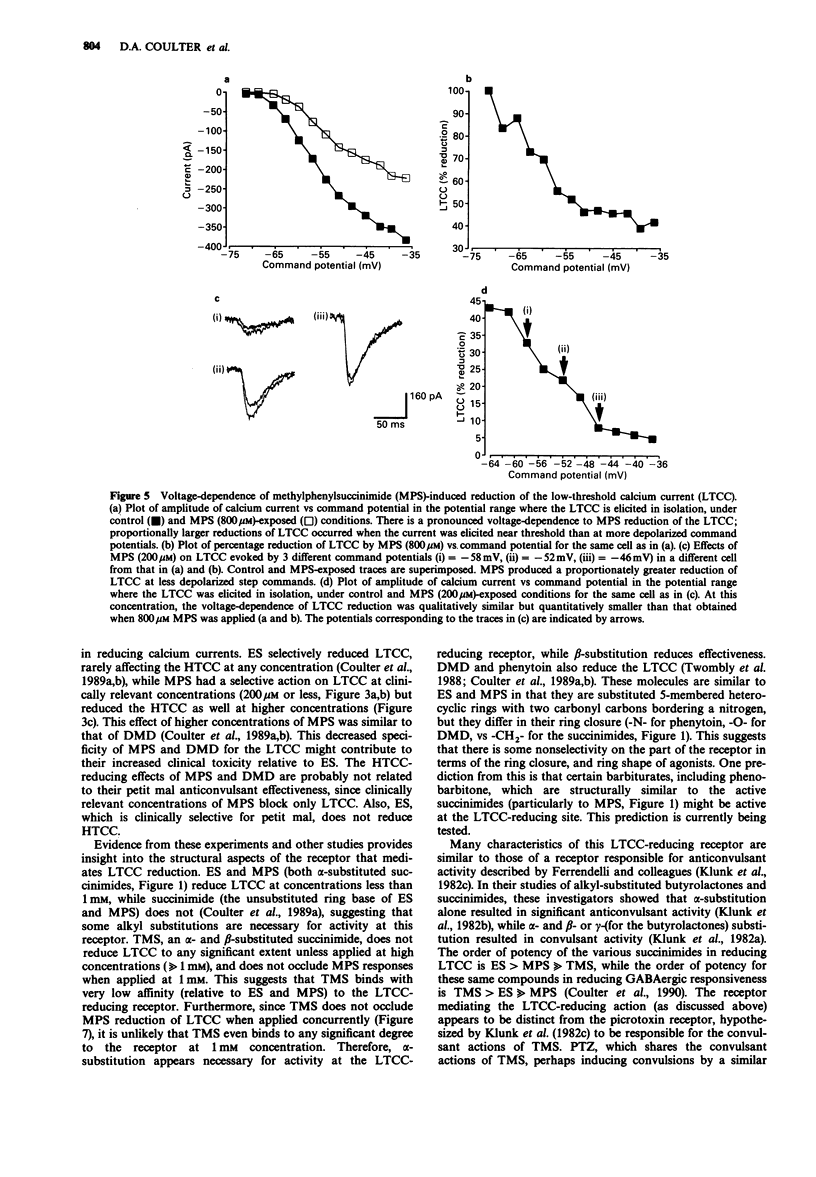
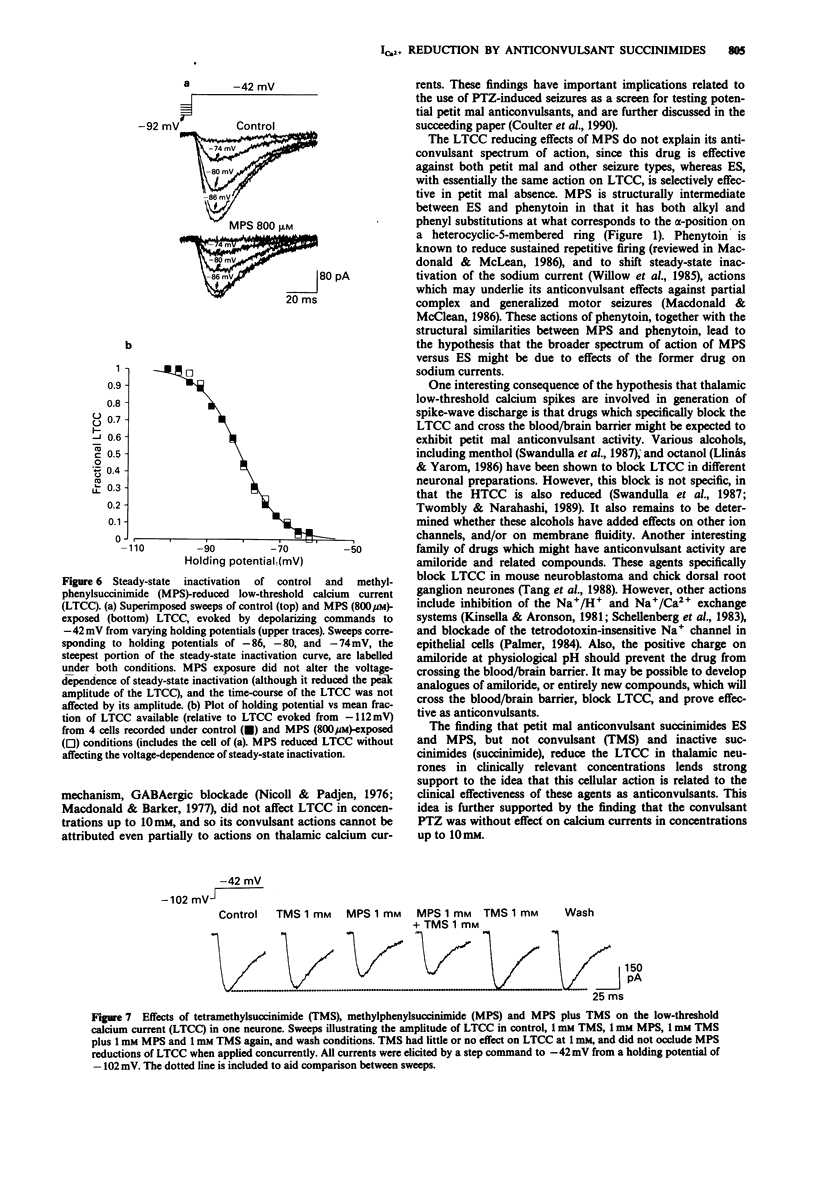
1. Succinimide derivatives can be either convulsant (tetramethylsuccinimide (TMS)), or anticonvulsant (ethosuximide (ES); alpha-methyl-alpha-phenylsuccinimide (MPS)). ES, an anticonvulsant succinimide, has previously been shown to block calcium currents of thalamic neurones, while the convulsant succinimide TMS blocks gamma-aminobutyric acid (GABA) responses in a similar fashion to the convulsant pentylenetetrazol (PTZ). 2. Using voltage-clamp techniques, we analysed the effects of the anticonvulsant succinimides ES and MPS and the convulsants TMS and PTZ on calcium currents of acutely isolated thalamic relay neurones of the rat. 3. MPS and ES reduced low-threshold calcium current (LTCC) in a voltage-dependent manner, without affecting steady-state inactivation. MPS was less potent than ES (IC50 of 1100 vs 200 microM) but greater in efficacy (100% maximal reduction vs 40% for ES). 4. PTZ had no effect on calcium currents, and TMS only reduced LTCC at very high concentrations, and did not occlude MPS effects when applied concurrently. 5. These results, which demonstrate that anticonvulsant, but not convulsant, succinimides block LTCC, provide additional support for the hypothesis that LTCC reduction is a mechanism of action of the anticonvulsant succinimides related to their effects in petit mal epilepsy.
Full text
PDF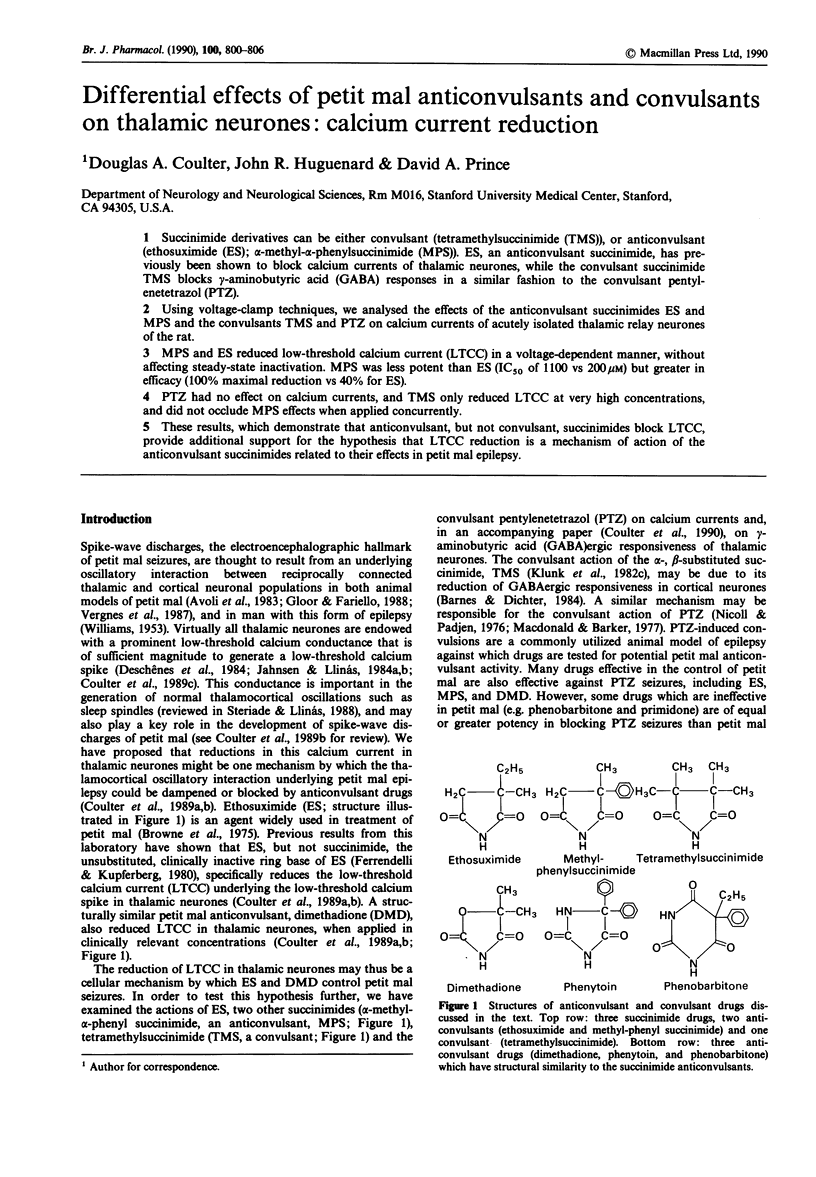


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avoli M., Gloor P., Kostopoulos G., Gotman J. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 1983 Oct;50(4):819–837. doi: 10.1152/jn.1983.50.4.819. [DOI] [PubMed] [Google Scholar]
- Barnes D. M., Dichter M. A. Effects of ethosuximide and tetramethylsuccinimide on cultured cortical neurons. Neurology. 1984 May;34(5):620–625. doi: 10.1212/wnl.34.5.620. [DOI] [PubMed] [Google Scholar]
- Browne T. R., Dreifuss F. E., Dyken P. R., Goode D. J., Penry J. K., Porter R. J., White B. G., White P. T. Ethosuximide in the treatment of absence (peptit mal) seizures. Neurology. 1975 Jun;25(6):515–524. doi: 10.1212/wnl.25.6.515. [DOI] [PubMed] [Google Scholar]
- Browne T. R., Feldman R. G., Buchanan R. A., Allen N. C., Fawcett-Vickers L., Szabo G. K., Mattson G. F., Norman S. E., Greenblatt D. J. Methsuximide for complex partial seizures: efficacy, toxicity, clinical pharmacology, and drug interactions. Neurology. 1983 Apr;33(4):414–418. doi: 10.1212/wnl.33.4.414. [DOI] [PubMed] [Google Scholar]
- CHEN G., WESTON J. K., BRATTON A. C., Jr Anticonvulsant activity and toxicity of phensuximide, methsuximide and ethosuximide. Epilepsia. 1963 Mar;4:66–76. doi: 10.1111/j.1528-1157.1963.tb05209.x. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989 Jul;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989 Jun;25(6):582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Differential effects of petit mal anticonvulsants and convulsants on thalamic neurones: GABA current blockade. Br J Pharmacol. 1990 Aug;100(4):807–813. doi: 10.1111/j.1476-5381.1990.tb14096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Specific petit mal anticonvulsants reduce calcium currents in thalamic neurons. Neurosci Lett. 1989 Mar 13;98(1):74–78. doi: 10.1016/0304-3940(89)90376-5. [DOI] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P., Fariello R. G. Generalized epilepsy: some of its cellular mechanisms differ from those of focal epilepsy. Trends Neurosci. 1988 Feb;11(2):63–68. doi: 10.1016/0166-2236(88)90166-x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Anticonvulsant properties of alpha, gamma, and alpha, gamma-substituted gamma-butyrolactones. Mol Pharmacol. 1982 Sep;22(2):438–443. [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Comparison of epileptogenic properties of unsubstituted and beta-alkyl-substituted gamma-butyrolactones. Mol Pharmacol. 1982 Sep;22(2):431–437. [PubMed] [Google Scholar]
- Klunk W. E., Covey D. F., Ferrendelli J. A. Structure-activity relationships of alkyl-substituted gamma-butyrolactones and succinimides. Mol Pharmacol. 1982 Sep;22(2):444–450. [PubMed] [Google Scholar]
- Macdonald R. L., Barker J. L. Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurones. Nature. 1977 Jun 23;267(5613):720–721. doi: 10.1038/267720a0. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., McLean M. J. Anticonvulsant drugs: mechanisms of action. Adv Neurol. 1986;44:713–736. [PubMed] [Google Scholar]
- Mody I., Salter M. W., MacDonald J. F. Requirement of NMDA receptor/channels for intracellular high-energy phosphates and the extent of intraneuronal calcium buffering in cultured mouse hippocampal neurons. Neurosci Lett. 1988 Oct 31;93(1):73–78. doi: 10.1016/0304-3940(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Padjen A. Pentylenetetrazol: an antagonist of GABA at primary afferents of the isolated frog spinal cord. Neuropharmacology. 1976 Jan;15(1):69–71. doi: 10.1016/0028-3908(76)90099-x. [DOI] [PubMed] [Google Scholar]
- Palmer L. G. Voltage-dependent block by amiloride and other monovalent cations of apical Na channels in the toad urinary bladder. J Membr Biol. 1984;80(2):153–165. doi: 10.1007/BF01868771. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Anderson L., Swanson P. D. Inhibition of Na+-Ca2+ exchange in rat brain by amiloride. Mol Pharmacol. 1983 Sep;24(2):251–258. [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Strong J. M., Abe T., Gibbs E. L., Atkinson A. J., Jr Plasma levels of methsuximide and N-desmethylmethsuximide during methsuximide therapy. Neurology. 1974 Mar;24(3):250–255. doi: 10.1212/wnl.24.3.250. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Carbone E., Schäfer K., Lux H. D. Effect of menthol on two types of Ca currents in cultured sensory neurons of vertebrates. Pflugers Arch. 1987 Jun;409(1-2):52–59. doi: 10.1007/BF00584749. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Presser F., Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988 Apr 8;240(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Twombly D. A., Yoshii M., Narahashi T. Mechanisms of calcium channel block by phenytoin. J Pharmacol Exp Ther. 1988 Jul;246(1):189–195. [PubMed] [Google Scholar]
- Vergnes M., Marescaux C., Depaulis A., Micheletti G., Warter J. M. Spontaneous spike and wave discharges in thalamus and cortex in a rat model of genetic petit mal-like seizures. Exp Neurol. 1987 Apr;96(1):127–136. doi: 10.1016/0014-4886(87)90174-9. [DOI] [PubMed] [Google Scholar]
- Wilder B. J., Buchanan R. A. Methsuximide for refractory complex partial seizures. Neurology. 1981 Jun;31(6):741–744. doi: 10.1212/wnl.31.6.741. [DOI] [PubMed] [Google Scholar]
- Willow M., Gonoi T., Catterall W. A. Voltage clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol Pharmacol. 1985 May;27(5):549–558. [PubMed] [Google Scholar]


